Ambient Cistern
ABSTRACT
OBJECTIVE: We used microscopy to conduct qualitative and quantitative analysis of 4 surgical approaches commonly used in the surgery of the ambient cistern: infratentorial supracerebellar (SC), occipital interhemispheric, subtemporal (ST), and transchoroidal (TC). In addition, we performed a parahippocampal gyrus resection in the ST context.
METHODS: Each approach was performed in 3 cadaveric heads (6 sides). After the microscopic anatomic dissection, the parahippocampal gyrus was resected through an ST approach. The qualitative analysis was based on anatomic observation and the quantitative analysis was based on the linear exposure of vascular structures and the area of exposure of the ambient cistern region.
RESULTS: The ST approach provided good exposure of the inferior portion of the cistern and of the proximal segments of the posterior cerebral artery. After the resection of the parahippocampal gyrus, the area of exposure improved in all components, especially the superior area. A TC approach provided the best exposure of the superior area compared with the other approaches. The posterolateral approaches (SC/occipital interhemispheric) to the ambient cistern region provided similar exposure of anatomic structures. There was a significant difference (P < 0.05) in linear exposure of the posterior cerebral artery when comparing the ST/TC and ST/SC approaches.
CONCLUSIONS: This study has demonstrated that surgical approaches expose dissimilarly the different regions of the ambient cistern and an approach should be selected based on the specific need of anatomic exposure.
INTRODUCTION
The ambient cistern region is a unique cerebral compartment and presents a challenge to the neurosurgeon because of its deep location, surrounding vital structures, narrow boundaries, and complex tridimensional anatomy. Several surgical approaches with modifications and combinations have been described to access the ambient cistern region and the posterior portion of the posterior cerebral artery (PCA). However, selecting the appropriate surgical route remains controversial and requires precise understanding of the limitations of each approach as well as the anatomy of the region.1-9
We conducted a qualitative and quantitative analysis of 4 surgical approaches used in the surgery of the ambient cistern: infratentorial supracerebellar (SC), occipital interhemispheric (OI), subtemporal (ST), and transchoroidal (TC). In addition, we performed resection of the parahippocampal gyrus when carrying out the ST approach with parahippocampal gyrus resection (STh). We have studied the role of endoscope assistance in other work using the same surgical approaches. The goals of the present study were to highlight the microanatomy of the ambient cistern region from different perspectives, objectively compare the approaches in terms of anatomic exposition using a computerized tracking system, and discuss the strategies for approaching lesions in this area.
METHODS
Three cadaveric heads (6 sides), without obvious intracranial disease, fixed in formalin and perfused with colored silicone, were included in this study. Before dissection, the specimens were rigidly fixed in a Mayfield head holder (Ohio Medical Instrument Co., Cincinnati, OH, USA) in a position that recreated surgical positioning. The procedures were performed using standard microsurgical and powered instruments and an operating microscope. Surgical Approaches The detailed surgical techniques for each approach have been well described and were performed as previously described in the literature.10–13
Suboccipital and occipital craniotomies were performed with preservation of the bone overlying the transverse sinus. The SC approach was followed by the OI approach, providing a posterior route to the ambient cistern region.
On the same side of the cadaver head, a low-set temporal craniotomy provided access to the ST and TC approaches. The ST approach involves placing the sagittal suture parallel to the floor with the vertex angled inferiorly to allow for maximal visualization along the tentorial surface to the ambient cistern. The arachnoid adhesions connecting the mesial temporal lobe to the tentorial edge were sharply dissected and removed to expose the underlying structures.
For the TC approach, we used the inferior temporal sulcus to access the temporal horn of the lateral ventricle and open the choroidal fissure between the hippocampal fimbria and the choroid plexus. The parahippocampal gyrus was resected through an ST approach to improve the exposition of the ambient cistern region.
Ambient Cistern Anatomy
The ambient cistern is a complex arachnoid compartment that is shaped like a letter C around the parahippocampal gyrus if viewed from a coronal perspective. It extends from the posterior margin of the crural cistern to the lateral edge of the midbrain colliculi.14 Some investigators6 consider the crural cistern as part of the anterior ambient cistern because they did not observe a definite border or separation between the 2 arachnoid compartments. We defined the ambient cistern region15 as the area that is limited anteriorly by the posterior lateral surface of the cerebral peduncle; medially by the lateral surface of the midbrain; laterally by the tentorial edge, parahippocampal gyrus, fimbria of the fornix, and choroidal fissure; and superiorly by the pulvinar of the thalamus, lateral geniculate body, and optic tract.
The region contains the anterior choroidal artery (AChA), the P2 and P3 segments of the PCA with their branches, the basal Rosenthal vein (BRV), and, infrequently, a segment of the superior cerebellar artery.
The AChA runs along the roof of the cistern and enters the choroidal fissure between the pial layers of the peduncle and the uncus, which fuse to form the tela choroidea.16–18
The P2 segment of the PCA can be subdivided into P2a and P2p, which are bordered by the posterior edge of the peduncle. The P2a begins at the junction of the posterior communicating artery and courses through the anterior ambient or crural cistern along the upper surface of the anterior perimesencephalic membrane. The P2p begins at the posterior border of the anterior ambient or crural cistern and ends at the lateral edge of the midbrain colliculi. The P2p often courses superiorly and laterally within the ambient cistern to lie on the superior surface of the parahippocampal gyrus. The P3 segment proceeds posteriorly from the posterior edge of the ambient cistern into the quadrigeminal cistern.19
The medial posterior choroidal artery (PChA) typically originates as a single trunk from the P1 or P2 and courses through the ambient cistern. It travels inferior and medial to the PCA through the crural and ambient cisterns and turns medially to enter the quadrigeminal cistern. The lateral PChAs arise most commonly from the P2p as 1 or several branches, course laterally along the upper edge of the parahippocampal gyrus within the ambient cistern, and pass through the choroidal fissure to enter the posterior part of the temporal horn and atrium.20–23
The BRV passes around the upper midbrain and drains the walls of the ambient cistern. It exits the ambient cistern and enters the arachnoid envelope over the pineal region to join the great vein or internal cerebral vein.24
Evaluation of Exposure
The qualitative analysis was performed based on anatomic observations of the surgical exposure and limitations of the different approaches during cadaveric dissections.
The quantitative analysis was based on the linear exposure of vascular structures and the area of exposure of the ambient cistern region. We used the Optotrak 3020 system (Northern Digital, Waterloo, Canada) with a 6-marker digitizing probe and accompanying software for data collection. The head was fixed rigidly in a 3-point head holder to ensure that it remained in the same Cartesian coordinate system as the Optotrak. A computer connected to the Optotrak system stored data files in the form of x, y, and z coordinates (in millimeters) of each vertex. The retractor was secured firmly to prevent measurement errors, whereas the points were located spatially. A data point was acquired by touching the tip of the digitizing probe to the anatomic points of interest while its position was recorded with cameras.
We evaluated the extent of exposure of the PCA, medial PChA, and BRV in each approach.
The area of surgical exposure was measured and calculated as in previous studies.25–27 We divided the total area into 3 components: anterior, medial, and superior areas. In each component, 4 points were selected forming a quadrangle or 2 triangles with a common side. The area of exposure of the anterior, medial, and superior regions was measured by summing the areas formed by the 2 triangles. The areas of the triangles were calculated as half the magnitude of the vector cross-product of any 2 vectors forming 2 sides of a triangle.
The anterior component was defined as the region immediately lateral to the cerebral peduncle and anterior to the lateral mesencephalic sulcus. The points selected on the anterior area were 1) the most inferior and anterior point of surgical exposure (point 1); 2) the most superior and anterior point of surgical exposure (point 2); 3) the most inferior point at the lateral mesencephalic sulcus (point 3); and 4) the most superior point at the lateral mesencephalic sulcus (point 4) (Figures 1 and 2).
Figure 1. Inferior perspective of the ambient cistern region. The figure demonstrates the landmark points used to evaluate the area of exposure of the superior component. Int. Ped. C, Internal peduncular cistern; Crural C., Crural cistern; Amb. C., Ambiens cistern; Quad. C., Quadrigeminal cistern; Splenium, Splenium; Pinela, Pineal gland; Parahip. G., Parahippocampal gyrus; Lat Mes S, Lateral mesencephalic sulcus; Cer. Ped., Cerebral Peduncle; Mam. Bd, Mamillary bodies; Uncus, Uncus; Opt. Tr, Optic tract; Chiasm, Optic chiasm. (Image courtesy of AL Rhoton, Jr.)
Figure 2. Lateral perspective of the ambiens cistern region. The figure illustrates the landmark points used to evaluate the area of exposure at the anterior and medial components. PCA, posterior cerebral artery; Post Ch Aa, posterior choroidal arteries; Lat Mes S, lateral mesencephalic sulcus; Basal V, basal vein; Sup Pt V, superior petrosal vein; VII, facial nerve; VI, abducens nerve; SCA, superior cerebellar artery; BA, basilar artery; AICA, anterior inferior cerebellar artery. (Image courtesy of AL Rhoton, Jr.)
The medial component was defined as the region immediately lateral to the mesencephalon and posterior to the lateral mesencephalic sulcus. The points selected on the medial area were 1) the most inferior point at the lateral mesencephalic sulcus (point 3); 2) the most superior point at the lateral mesencephalic sulcus (point 4); 3) the most inferior and posterior point of surgical exposure (point 5); and 4) the most superior and posterior point of surgical exposure (point 6) (Figures 1 and 2).
The superior component was defined as the region on the roof of the ambient cistern, which corresponds to the pulvinar of the thalamus, lateral geniculate body, and optic tract. The points selected on the superior region were 1) the most anterior and lateral point on the roof of the cistern (point 7); 2) the most anterior medial point on the roof of the cistern (point 8); 3) the most posterior and lateral point of the roof of the cistern (point 9); and 4) the most posterior medial point of the roof of the cistern (point 10) (Figures 1 and 2).
Statistical Analysis
Differences in surgical areas of exposure and linear exposure were analyzed by 1-way repeated analysis of variance followed by the Holm-Sidak method and the Dunn method. In all cases, a P value less than 0.05 was considered significant.
RESULTS
Qualitative Analysis
ST Approach and Resection of the Parahippocampal Gyrus
The ST approach provided a lateral inferior route to the ambient cistern region. The arachnoid dissection and upward retraction of the temporal lobe exposed the lower half or two thirds of the ambient cistern where the basilar tip, part of the cisternal segment of the AChA, the medial PChA, and the P1 and P2a segments of the PCA were exposed. The proximal P2p segment of the PCA could be accessed; however, further brain retraction was needed to expose the distal P2p because it is directed superiorly and laterally, hidden by the parahippocampal gyrus. The BRV was partially exposed. The lateral PChA and P3 segment of the PCA were inaccessible through this approach.
The main limitations of surgical exposure of the ambient cistern region were the vein of Labbé and the parahippocampal gyrus. The vein of Labbé limited temporal lobe retraction and surgical exposure of the superior posterior portion of the ambient cistern. The medial edge of the parahippocampal gyrus blocked the access to the upper one third of the cistern.
The resection of the parahippocampal gyrus improved the exposure of the superior area of the ambient cistern. Consequently, better access to the BRV and the P2p segment of the PCA was obtained.
TC Approach
The transtemporal TC approach provided a lateral superior route to the ambient cistern region. Opening the choroidal fissure between the hippocampal fimbria and the choroid plexus easily exposed the ambient cistern, where the first structures encountered were the BRV and the lateral geniculate body in the roof of the cistern.
The caudal retraction of the hippocampal formation provided access to the upper one third of the ambient cistern, where the P2p and proximal P3 were viewed. The crural cistern could not be reached through the choroidal fissure unless an anterior incision from the inferior choroidal point was made. The AChA and the lateral PChA branches were exposed as they entered the ventricular system through the choroidal fissure.
Further caudal retraction and dissection provided better inferior exposure of the ambient cistern as well as exposure of the posterior crural and anterior quadrigeminal cisterns. The limitation of this approach is access to the medial PChA (located below the PCA) and the cisternal segments of the AChA, P1, and P3.
Infratentorial SC Approach and OI Approach. The SC and the OI approaches provided a posterior lateral route to the ambient cistern region. Despite the superior trajectory of the OI and the inferior trajectory of the SC, the exposure of anatomic landmarks was similar for both approaches.
The P3 and P2p segments were readily accessible. The distal P2a segment could be exposed and the proximal segments of the PCA were inaccessible through these approaches. Similarly, the distal segments of the medial PChA (ambient and quadrigeminal cisterns) could be exposed.
The AChA could not be reached because it entered the ventricle at the inferior choroidal point. The lateral PChA was inaccessible because it was directed toward the temporal horn above the parahippocampal gyrus.
Quantitative Analysis
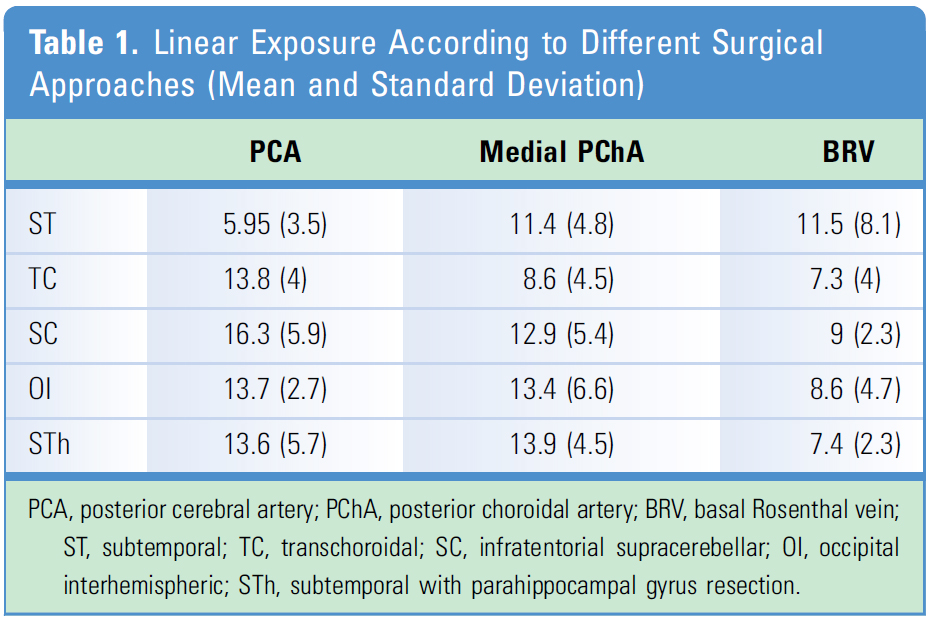
Linear Exposure. The microscopic linear exposure of the PCA was ST = 5.95 +/- 3.52 mm, TC = 13.8 +/- 4.04 mm, SC = 16.37 +/- 5.93 mm, OI = 13.7 +/- 2.75 mm, and STh = 13.6 +/- 5.78 mm. For the medial PChA, we found ST = 11.47 +/- 4.81 mm, TC = 8.6 +/- 4.54mm, SC = 12.93 +/- 5.44 mm, OI 13.42 +/- 6.64 mm, and STh = 13.95 +/- 4.51mm. It was not possible to expose the BVR in 3 sides through the ST approach and in 2 sides of the TC approach. The linear exposure of the BRV was ST = 11.5 +/- 8.3 mm, TC = 7.34 +/- 4.2 mm, SC = 9.03 +/- 2.33 mm, OI = 8.6 +/- 4.7 mm, and STh = 7.48 +/- 2.3 mm (Table 1 and Figure 3).
Linear Exposure
Figure 3. Linear exposure of the posterior cerebral artery (PCA), medial posterior choroidal artery (PChA) and basal Rosenthal vein (BRV) through the different surgical approaches. OI, occipital interhemispheric; SC, infratentorial supracerebellar; ST, subtemporal; STh, subtemporal with parahippocampal gyrus resection; TC, transchoroidal. (Image courtesy of AL Rhoton, Jr.)
When we statistically evaluated the differences in linear exposure, significance was found only in linear exposure for PCA between the ST and TC approaches and the ST and SC approaches (Table 1 and Figure 3).
Area of Exposure
The results for area of exposure are as follows. superior area ST = 7.9 +/- 11.1 mm2, TC= 65.8 +/- 24.5 mm2, SC: 24.2 +/- 15.9 mm2, OI = 35.1 +/- 31.1 mm2, and STh = 60.3 +/- 19.9 mm2. The medial area was ST = 54.3 +/- 17.3 mm2, TC = 46.8 +/- 30.5 mm2, SC = 55.5 +/- 27.5 mm2, OI = 44.6 +/- 13.8 mm2, and STh = 81.0 +/- 10.5 mm2. The anterior area exposure was ST = 42 +/- 9.9 mm2, TC = 45.6 +/- 18.3 mm2, SC = 48.7 +/- 15.3 mm2, OI = 42.6 +/- 14.3 mm2, and STh = 69.6 +/- 9.6 mm2. The sum of all area components resulted in a total area of exposure for each approach of on the ST = 104.8 +/- 19.8 mm2, TC = 158.2 +/- 51.2 mm2, SC = 137.2 +/- 47.7 mm2, OI = 118.5 +/- 40.0 mm2, and STh = 210.5 +/- 31.9 mm2 (Figure 4). No statistical differences were found in areas of exposure among ST, TC, SC, and OI. However, statistical differences were evident for the anterior area of exposure between STh and ST and between STh and OI. In addition, statistical differences also were found in total area of exposure between STh and ST, between STh and SC, and between STh and OI.
Figure 4. Areas of exposure (superior, medial, anterior, and total) through the different surgical approaches. OI, occipital interhemispheric; SC, infratentorial supracerebellar; ST, subtemporal; STh, subtemporal with parahippocampal gyrus resection; TC, transchoroidal. (Image courtesy of AL Rhoton, Jr.)
DISCUSSION
The complex tridimensional anatomy and density of vital structures make the ambient cistern region a unique and challenging cerebral compartment that cannot be fully exposed by a single approach. This is the reason that selecting the appropriate surgical route remains controversial.1–9
The different approaches described in this study exposed dissimilarly the ambient cistern region. The ST approach provided good exposure of the inferior portion of the cistern and of the proximal segments of the PCA. The main disadvantage is access to the upper portion of the cistern, which is limited by the parahippocampal gyrus and temporal lobe retraction (the vein of Labbé). It prevented the exposure of the BRV on 3 sides.
After the resection of the parahippocampal gyrus, the area of exposure improved in all components, especially the superior area. Also, the linear exposure of the medial PChA and PCA (improved exposure of the P2p segment) was greater compared with the ST approach. Despite the smaller value on the average linear exposure of the BRV, it was possible to expose the BRV on all sides on the STh approach.
The TC approach provided the best exposure of the superior area compared with the other approaches. The major drawbacks to the TC approach are the need to perform a corticectomy on the temporal lobe and the exposure of the inferior segment of the cistern. The linear exposure of the medial PChA was the smallest of all approaches, because the artery is located below the PCA. In addition, despite the good exposure of the superior area, it was not possible to expose the BRV in 2 sides through this approach because of anatomic variations. Venous anatomy interferes directly with the surgical exposure because it may limit visualization of critical structures. The complexity of vein anatomy may be partially responsible for the results we found, mainly relating to the OI and SC approaches. However, because we specifically studied the ambient cistern region, we limited our measurements to the linear exposure of BRV, the main vein structure in the ambient cistern.
The posterolateral approaches (SC and OI) to the ambient cistern region provided similar exposure of anatomic structures. Overall, these routes had better linear exposure than the ST and TC approaches and similar linear exposure to the STh approach.
Despite the difficulty of accessing the proximal PCA, the anterior component of the area of exposure had values close to those for the ST and TC approaches. The main disadvantages of the SC and OI approaches are the long working distance to the ambient cistern region and inability to control the proximal PCA. Our results have demonstrated that selecting TC, STh, or SC instead of ST may improve linear exposure of PCA. This fact may be relevant in planning surgical approaches to treat vascular or tumoral lesions involving the PCA in the ambient cistern region. When we statistically evaluated the differences in linear exposure, significance was found only in linear exposure between the ST and TC approaches and the ST and SC approaches. These results demonstrated that the resection of the parahippocampal gyrus does not increase areas of exposure in the ST technique compared with the TC approach. Using a TC approach instead of ST may obviate parahippocampal gyrus resection.
STUDY LIMITATIONS
The response of formalin-fixed cadaveric tissue does not duplicate the response of tissue in vivo. Such factors can affect the results in terms of retraction or shrinkage of structures. It is difficult to predict how these differences may have influenced the final results, although we made every effort to maintain consistency in retraction, among other variables. The advantage of gravity in altering head position, cerebrospinal fluid drainage, venous congestion, and brain tissue elasticity cannot be reproduced.
We do not consider the number of the heads a limitation. Ethical and economic issues worldwide have increasingly restricted the number of available specimens and several studies have been reported with a similar number of heads with no detriment to the statistical significance.25–27
The selection of any surgical technique should be based primarily on the anatomic exposure that it affords. However, surgical experience and familiarity with using a particular approach also have a decisive role. Nonetheless, objective and anatomic data such as provided by this study help us to understand the advantages and limitations of different approaches to the same region and may support a surgeon’s choice, which should not be based only on intuition and personal impressions.
CONCLUSIONS
This study has demonstrated that surgical approaches expose dissimilarly the different regions of the ambient cistern. There was statistical difference in linear exposure of the PCA when comparing the ST and TC approaches and ST and SC approaches. In addition, our data demonstrated that the TC approach may obviate parahippocampal gyrus resection in an ST approach scenario. The approach selection should be based on the specific need of anatomic exposure.
Contributors: Eberval Gadelha Figueiredo, André Beer-Furlan, Leonardo C. Welling, Eduardo C. Ribas, Marcelo Schafranski, Neil Crawford, Manoel J. Teixeira, Albert L. Rhoton, Jr, Robert F. Spetzler, and Mark C. Preul
Content from Figueiredo EG, Beer-Furlan A, Welling LC, Ribas EC, Schafranski M, Crawford N, Teixeira MJ, Rhoton AL, Jr, Spetzler RF, Preul MC. Microsurgical approaches to the ambient cistern region: an anatomic and qualitative study. World Neurosurg 2016;87:584–590. doi.org/10.1016/j.wneu.2015.10.063.
The Neurosurgical Atlas is honored to maintain the legacy of Albert L. Rhoton, Jr, MD.
References
- Gerber CJ, Dwyer G, Evans BT. An alternative surgical approach to aneurysm of the posterior cerebral artery. Neurosurgery. 1993;32:928-931.
- Gerber CJ, Dwyer GN. A review of the management of 15 cases of aneurysms of the posterior cerebral artery. Br J Neurosurg. 1992;6:521-527.
- Heros RC. Brain resection for exposure of deep extracerebral and paraventricular lesions. Surg Neurol. 1990;34:188-195.
- Ikeda K, Shoin K, Mohri M, Kijma T, Someya S, Yamashita J. Surgical indications and microsurgical anatomy of the transchoroidal fissure approach for lesions in and around the ambient cistern. Neurosurgery. 2002;50:1114-1120.
- Orita T, Tsurutani T, Kitahara T. P2 aneurysm approached via the temporal horn: technical case report. Neurosurgery. 1997;41:972-974.
- Qi ST, Fan J, Zhang XA, Pan J. Reinvestigation of the ambient cistern and its related arachnoid membranes: an anatomical study. J Neurosurg. 2011;115:171-178.
- Seoane ER, Tedeschi H, de Oliveira EP, Siqueira MG, Calderon GA, Rhoton AL Jr. Cerebral artery aneurysms: a proposed new surgical classification. Acta Neurochir (Wien). 1997;139:325-331.
- Teresaka S, Sawamura Y, Kamiyama H, Fukushima T. Surgical approaches for the treatment of aneurysms on the P2 segment of the posterior cerebral artery. Neurosurgery. 2000;47:359-365.
- Yonekawa Y, Imhof H, Taub E, Curcic M, Kaku Y, Roth P, et al. Supracerebellar transtentorial approach to posterior temporomedial structures. J Neurosurg. 2001;94:339-345.
- Siwanuwatn R, Deshmukh P, Zabramski JM, Preul MC, Spetzler RF. Microsurgical anatomy and quantitative analysis of the transtemporal transchoroidal fissure approach to the ambient cistern. Neurosurgery. 2005;57:228-235; discussion: 228-235.
- Spallone A, Makhmudov UB, Mukhamedjanov DJ, Tcherekajev VA. Petroclival meningioma: an attempt to define the role of skull base approaches in their surgical management. Surg Neurol. 1999;51:412-420.
- Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg. 1971;35:197-202.
- Tedeschi H, Rhoton AL Jr. Lateral approaches to the petroclival region. Surg Neurol. 1994;41:180-216.
- Nagata S, Rhoton AL Jr, Barry M. Microsurgical anatomy of the choroidal fissure. Surg Neurol. 1988;30:3-59.
- Ulm AJ, Tanriover N, Kawashima M, Campero A, Bova FJ, Rhoton A Jr. Microsurgical approaches to the perimesencephalic cisterns and related segments of the posterior cerebral artery: comparison using a novel application of image guidance. Neurosurgery. 2004;54:1313-1327.
- Inoue K, Seker A, Osawa S, Alencastro LF, Matsushima T, Rhoton AL Jr. Microsurgical and endoscopic anatomy of the supratentorialarachnoidal membranes and cisterns. Neurosurgery. 2009;65:644-665.
- Rhoton AL Jr, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979;12:171-187.
- Rhoton AL Jr. The supratentorial arteries. Neurosurgery. 2002;51 (4 Suppl):S53-S120.
- Zeal A, Rhoton AL Jr. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg. 1978;48:534-559.
- Fujii K, Lenkey C, Rhoton AL Jr. Microsurgical anatomy of the choroidal arteries: lateral and third ventricles. J Neurosurg. 1980;52:165-188.
- Milisavljevic MM, Marinkovic SV, Gibo H, Puskas LF. The thalamogeniculate perforators of the posterior cerebral artery: the microsurgical anatomy. Neurosurgery. 1991;28:523-529.
- Rhoton AL Jr. The lateral and third ventricles. Neurosurgery. 2002;51 (4 Suppl):S207-S271.
- Timurkaynak E, Rhoton AL Jr, Barry M. Microsurgical anatomy and operative approaches to the lateral ventricles. Neurosurgery. 1986;19:685-723.
- Rhoton AL Jr. The cerebral veins. Neurosurgery. 2002;51 (4 Suppl):S159-S205.
- Figueiredo EG, Deshmukh P, Nakaji P, Crusius MU, Teixeira MJ, Spetzler RF, et al. Anterior selective amygdalohippocampectomy: technical description and microsurgical anatomy. Neurosurgery. 2010;66 (3 Suppl Operative):45-53.
- Figueiredo EG, Deshmukh P, Nakaji P, Crusius MU, Crawford N, Spetzler RF, et al. The minipterional craniotomy: technical description and anatomic assessment. Neurosurgery. 2007;61:256-264.
- Figueiredo EG, Deshmukh P, Zabramski JM, Preul MC, Crawford N, Spetzler RF. The pterional transylvian approach: an analytical study. Neurosurgery. 2006;59 (4 Suppl 2):ONS263-ONS269; discussion: ONS269.
Please login to post a comment.