Glioblastoma
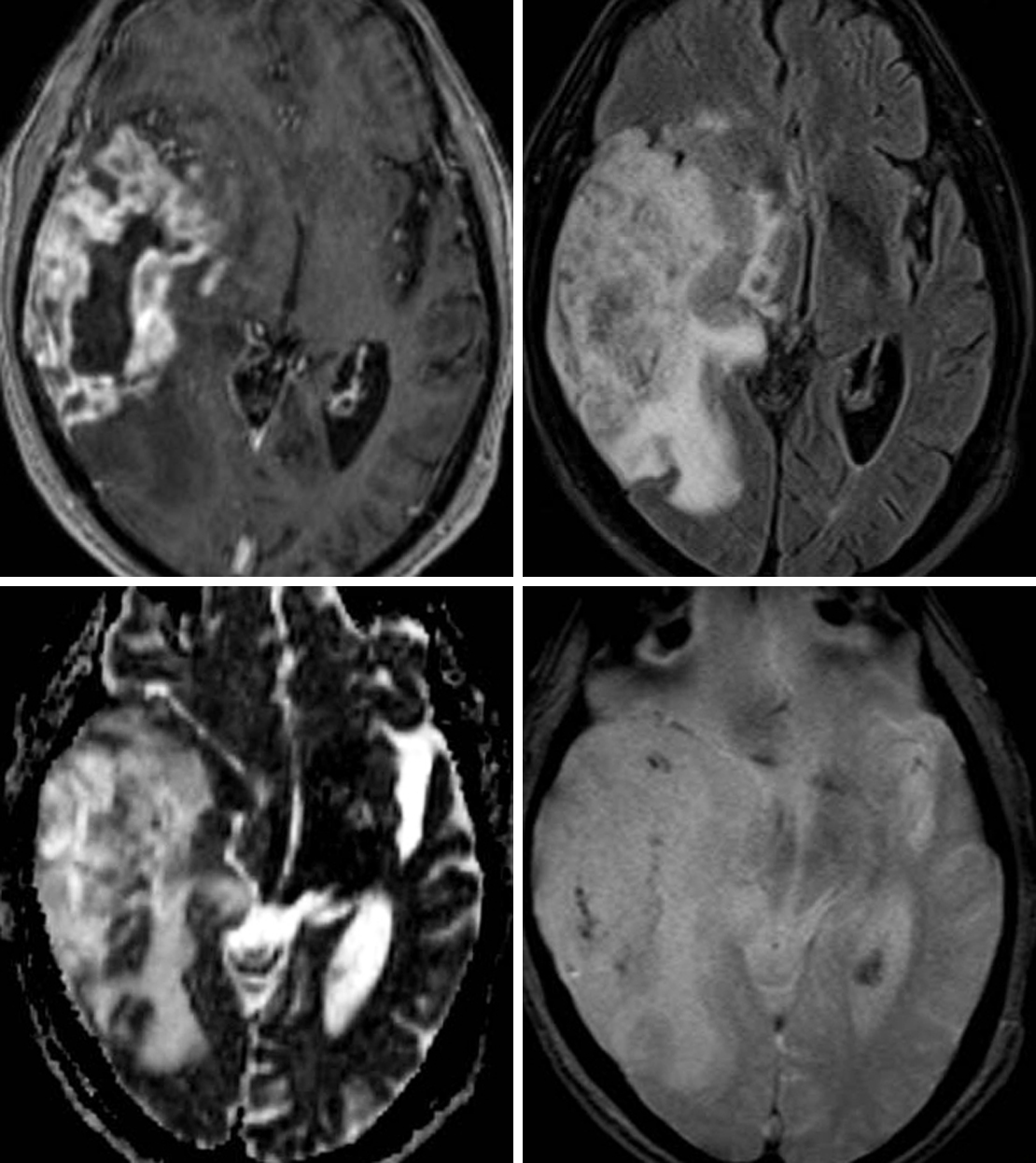
Figure 1: (Top Left) The avid peripheral enhancement and central necrosis seen in this lesion on postcontrast T1WI is typical of a GBM. (Top Right) Infiltrative FLAIR hyperintensity extending beyond the enhancing margins usually represents a combination of edema and nonenhancing infiltrative tumor. (Bottom Left) Areas of low signal intensity in regions of known tumor on ADC imaging usually indicate hypercellularity. (Bottom Right) Hemorrhagic foci are also not uncommonly seen on GRE or SWI, as in this patient's GBM.
BASIC DESCRIPTION
- Malignant, rapidly enlarging astrocytic tumor with microvascular proliferation and necrosis
- Most common primary intracranial neoplasm
PATHOLOGY
- WHO grade IV
- Necrosis and microvascular proliferation are characteristic features
- Develops de novo (primary) or from malignant dedifferentiation of anaplastic astrocytomas (secondary)
- Uncommonly secondary to previous radiation
- Primary glioblastoma (GBM) more aggressive, more common in elderly patients
- Secondary GBM more common in younger patients
- Associated with neurofibromatosis type 1 (NF-1), Turcot, Li-Fraumeni, and Maffucci syndromes and Ollier disease
CLINICAL FEATURES
- All ages affected (ages 45–75 years most common)
- Slight male gender predilection
- Median survival <1 year
- Better prognosis with younger age, greater extent of resection, and O(6)-methylguanine-DNA methyltransferase (MGMT)-positive genetics
- Presenting symptoms depend on tumor location
- Seizures, focal neurologic deficits
- Treatment: tumor debulking, radiotherapy, chemotherapy (temozolomide, antiangiogenesis agents)
IMAGING
- General
- Poorly defined, infiltrating supratentorial white matter mass
- Neoplastic cells extend beyond areas of signal abnormality
- Frontal, temporal, and parietal lobes most common
- Brainstem and cerebellum more common in children
- Usually solitary but can be multifocal (synchronous tumors) in up to 20% of cases
- Neovascularity and necrosis are common features
- Thick, irregularly enhancing rind with central necrosis
- Cysts, hemorrhage, fluid-debris levels, and vascular flow voids are common features
- Commonly spreads along white matter tracts and crosses midline via the corpus callosum, anterior and posterior commissures (“butterfly” glioma)
- Occasional cerebrospinal fluid dissemination
- Poorly defined, infiltrating supratentorial white matter mass
- CT
- Irregular hypodense to isodense mass with central hypodensity (necrosis)
- Surrounding vasogenic edema/tumor with mass effect on adjacent structures
- Radiodense hemorrhage could be present, calcification rare
- Irregular, heterogeneous rind of enhancement on contrast-enhanced CT
- MRI
- T1WI: irregular, hypointense white matter mass; areas of hyperintensity could represent subacute hemorrhage
- T2WI: heterogeneously hyperintense; surrounding vasogenic edema and tumor infiltration; presence of flow voids suggests neovascularity
- FLAIR: heterogeneously hyperintense
- T2*GRE: susceptibility artifact related to blood products/hemorrhage
- DWI: diffusion restriction reflecting hypercellularity is common in solid tumor components
- T1WI+C: thick, irregular rind of peripheral enhancement; enhancement can be ring, nodular, homogenous, or patchy
- MRS/MR perfusion: decreased NAA and myoinositol, increased Cho, lipid/lactate peak (1.3 ppm) indicating anaerobic metabolism of necrosis, increased relative cerebral blood volume (rCBV) compared with rCBV associated with lower-grade astrocytomas
- Diffusion tensor imaging (DTI) tractography and functional MRI (fMRI) may assist in surgical planning
IMAGING RECOMMENDATIONS
- MRI with contrast; MRS, MR perfusion, DTI, and fMRI are often useful adjuncts
For more information, please see the corresponding chapter in Radiopaedia.
Contributor: Rachel Seltman, MD
References
Arevalo-Perez J, Peck KK, Young RJ. Dynamic contrast-enhanced perfusion MRI and diffusion-weighted imaging in grading of gliomas. J Neuroimaging 2015;25:792–798. doi.org/10.1111/jon.12239.
Blasel S, Zagorcic A, Jurcoane A, et al. Perfusion MRI in the evaluation of suspected glioblastoma recurrence. J Neuroimaging 2016;26:116–123. doi.org/10.1111/jon.12247.
Calli C, Kitis O, Yunten N, et al. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur J Radiol 2006;58:394–403. doi.org/10.1016/j.ejrad.2005.12.032.
Di Costanzo A, Trojsi F, Giannatempo GM, et al. Spectroscopic, diffusion and perfusion magnetic resonance imaging at 3.0 Tesla in the delineation of glioblastomas: preliminary results. J Exp Clin Cancer Res 2006;25:383–390.
Ideguchi M, Kajiwara K, Goto H, et al. MRI findings and pathological features in early-stage glioblastoma. J Neurooncol 2015;123:289–297. doi.org/10.1007/s11060-015-1797-y.
Koukourakis GV, Kouloulias V, Zacharias G, et al. Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article. Molecules 2009;14:1561–1577. doi.org/10.3390/molecules14041561.
Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95:190–198. doi.org/10.3171/jns.2001.95.2.0190.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. doi.org/10.1007/s00401-007-0243-4.
Osborn, AG, Salzman KL, Jhaveri MD. Diagnostic Imaging (3rd ed). Elsevier, Philadelphia, PA; 2016.
Miletic H, Niclou SP, Johansson M, et al. Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms. Expert Opin Ther Targets 2009;13:455–468. doi.org/10.1517/14728220902806444.
Please login to post a comment.