General Principles
Figure 1: Harvey Cushing is the father of modern neurosurgery. His contributions paved the way for the establishment of the safety of brain tumor surgery in the face of critics.
Craniotomy techniques for brain tumor surgery have evolved immensely because of advances in imaging and microsurgical techniques. The development of flexible operative corridors, cortical and subcortical mapping, and magnetic resonance imaging (MRI) navigation have all enhanced the ability of the surgeon to resect tumors that were previously deemed inoperable.
With these technological advancements, the emphasis on simply minimizing mortality has shifted toward minimizing morbidity while also achieving maximal resection to provide an optimal outcome for patients.
In this chapter, I review the general principles of brain tumor surgery. The specific tenets for resection for each pathology are covered in the dedicated tumor chapters.
Evaluation
A careful history and physical examination are necessary to verify symptoms and correlate imaging findings. It is important to rule out non-neoplastic causes of mass lesions. Examples of these include infectious sources such as toxoplasmosis, vascular events such as evolving infarction, inflammatory factors such as sarcoidosis, and autoimmune causes such as multiple sclerosis.
These lesions can mimic tumors on imaging and presentation, and lead to unnecessary operative intervention, placing the patient at an avoidable risk. The surgeon should be intimately familiar with subtle clinical and imaging findings that increase suspicion for these disorders in the differential diagnosis.
Figure 2: These images show examples of lesions that do not require resection as part of their treatment strategy. These images belong to three patients with multiple sclerosis. Note that the pattern of enhancement is faint in certain locations at the periphery of the lesion. The resultant extent of mass effect is minimal compared with the size of the lesion. Neoplasms frequently lead to more mass effect when they reach a large size.
Figure 3: The images of the top row belong to a patient who suffered from a seizure. The MR enhanced images demonstrate gyral enhancement pattern compatible with acute infarction and not a neoplasm. The rest of the images in the subsequent rows belong to another patient with hemiparesis who was thought to have an insular tumor. A careful review of these images advances the suspicion of an infarct as the T2 hyperintensity crosses the striatum (left lower image); this feature is not consistent with tumorous masses that respect the medial gray matter structures. Diffusion imaging (right lower photo) confirms the findings of infarction and avoids unnecessary operative intervention.
Some tumors are very amenable to radiation therapy and do not require surgical resection. Germinomas, lymphomas, cerebral multiple myeloma and leukemia masses should be considered in the differential diagnosis of select neoplastic masses and nonresective diagnostic strategies such as CSF studies or stereotactic biopsy attempted.
Multiplicity of the lesion also undermines the benefits of aggressive resection.
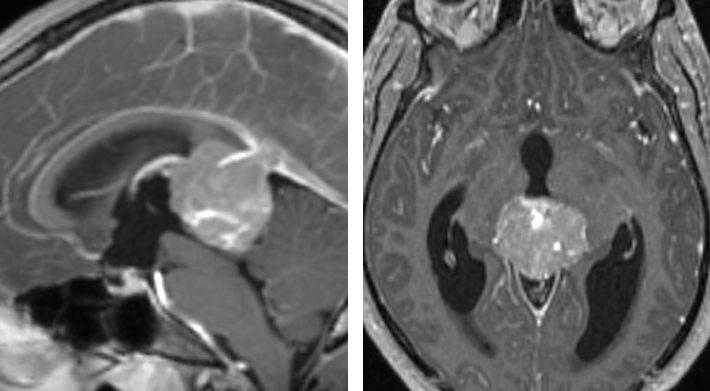
Figure 4: The MRI scan of this 18-year-old boy demonstrates a partially calcified pineal mass. The cerebrospinal fluid markers were consistent with a diagnosis of germinoma. Surgical resection is not warranted.
Most patients who are considered for tumor resection present with a computed tomography (CT) or MRI scan showing a mass lesion. An MRI, with its superior soft-tissue resolution, discloses important details about the pathoanatomy of the lesion(s) and its signal characteristics on different MR sequences. These data define the size, location, proximity to eloquent brain structures, and boundaries of the mass. The pattern of contrast enhancement can be very informative for reaching an appropriate preoperative differential diagnosis.
A CT scan may be necessary if there is bone or skull base involvement to determine the pattern of bony erosion, providing valuable data regarding the growth rate of the tumor. CT angiogram and catheter angiography can study the vascular anatomy and hemodynamic parameters in the surrounding vital vessels. Tumor embolization can be lifesaving with some tumors, such as glomus jugulare, hemangiopericytoma, and solid hemangioblastoma of the posterior fossa, when immediate tumor devascularization may not be feasible intraoperatively.
When evaluating the relevant imaging studies, the main consideration is to reach a conclusion to proceed with resection/biopsy versus other treatment modalities (chemotherapy or radiation) or observation and follow-up. If the boundaries of the mass are poorly defined, involve eloquent cortices/fibers or vital neurovascular structures, or the mass is multi-centric, a gross total resection may be impossible or provide the patient with suboptimal benefits. In these instances, other treatment modalities should be considered.
If the diagnosis is not reliably possible from imaging and other available data and nonresectable lesions are strongly considered in the differential diagnosis, the surgeon should pursue a stereotactic biopsy to direct the final treatment plan.
Preoperative Considerations
Before proceeding with operative intervention, other investigations may need to be performed depending on the patient’s other medical conditions and age. Patients who are older than 40 should obtain a preoperative chest x-ray, and patients who are older than 60 should have an electrocardiogram.
All patients should have a complete blood count, including a platelet count, as well as a basic metabolic panel. They should be “typed and crossed” if there is a reasonable possibility of the need for a perioperative blood transfusion. Patients with known easy bruising, a bleeding disorder, or who are taking anticoagulants for other medical conditions should also undergo prothrombin time (PT), partial thromboplastin time (PTT), and other coagulation tests as indicated by the patient’s history. Many patients with known or suspected malignancies with metastatic potential require a recent CT scan of the chest/abdomen/pelvis and possibly a bone scan. Neuro-oncological consultation is highly advised. Aggressive surgical strategy for patients with advanced systemic disease associated with less than 6-month lifespan is usually not justified.
Perioperative medications may be necessary for seizure prophylaxis or reducing vasogenic edema. Many patients present with seizures or are at high risk for postoperative seizures due to the location of their tumor (temporal lobe) and the extent of cortical dissection needed to remove the tumor. Keppra (levetiracetam) 20 mg/kg intravenous loading dose followed by 500-1000 mg q12 hours dosing is commonly prescribed for seizure prophylaxis.
Routine use of steroids is not recommended, although significant vasogenic edema may be reduced with dexamethasone 10 mg intravenous loading dose followed by 4 mg q6 hours maintenance dose in preparation for surgery.
Anesthetic Considerations
There are four major anesthetic concerns for the intraoperative management of intracranial masses: 1) cerebral perfusion pressure, 2) brain relaxation, 3) ”smooth” anesthesia, and 3) efficient transition to the postoperative period.
During the induction phase of anesthesia, reductions in the metabolic oxygen rate and intracranial pressure (ICP) are greatly beneficial; barbiturates may be used. After administration of a nondepolarizing muscle relaxant, narcotics may be given without the risk of increased ICP from chest rigidity.
Dexamethasone (10 mg) is typically given before induction to potentially assist with brain relaxation. An antibiotic such as cefazolin (1 g) is administered for infection prophylaxis. All patients should wear sequential compression stockings, and a receive a Foley catheter. A lumbar drain may be inserted in select patients to facilitate brain relaxation.
Maintenance of anesthesia is best obtained with volatile agents, preferably isoflurane, small doses of narcotics, and muscle relaxants. Isoflurane also decreases the cerebral metabolic rate of oxygen. Of note, isoflurane is known to slightly increase ICP.
In maintaining hemodynamic stability and cerebral perfusion pressure, only iso-osmolar fluids are administered to best preserve neuronal homeostasis. Fluids containing glucose should be avoided because hyperglycemia has been shown to negatively affect outcomes in patients with ischemic cerebral injury.
Patient Positioning
The overarching goal of positioning is to maintain the patient’s safety and comfort while optimizing exposure. A Mayfield clamp is firmly secured to the patient’s head with fixation points as far away from the incision as possible. It is important to be aware of any shunt catheters or burrholes when pinning to avoid puncturing the shunt or penetrating the burrhole, respectively.
Previous craniotomy sites are left unpinned to avoid sinking of the bone flap. Untraditional skull clamp placement in these situations should protect the patient’s eyes and ears. Slippage of the pin into the globe has been reported after the pin was placed too close to the orbital rim.
The vertical arms of the clamp should be perpendicular to the floor and the patient’s head rotated less than 45 degrees while the patient is in the supine position. A lateral or park-bench position is preferred for posterior scalp exposure.
Pillows and pads should be generously used to pad all the patient’s pressure points, preventing peripheral nerve injuries. The patient should be well secured to the operating room table, especially if the patient is obese and changes in patient positioning (tilting) are expected during the procedure to adjust the intradural working angles of the surgeon.
All of the intravenous lines and the lumbar drain catheter are rechecked to ensure their patency after finalizing the patient’s position and before prepping the skin. Shaving should be kept to a minimum and the planned incision should be marked on the scalp after confirming the tumor location with MRI navigation.
Accuracy of the navigation should be confirmed using standard landmarks: external auditory meatus, lateral and media canthus, and the nasion. Navigation can be misleading for lesions situated on the curvature of the skull; coronal, axial, and sagittal images should be used for accurate mapping of the tumor’s location. The location of the tumor, as guided by navigation, is confirmed with respect to surface landmarks (i.e., external auditory meatus and coronal suture, etc.).
Procedure
The nuances of technique for complication avoidance and management during craniotomy and exposure are discussed in the Cranial Approaches volume. During a craniotomy near the dural venous sinuses, I place two burr holes over the dural sinus, saving the bony cut near the sinus for last.
Thorough irrigation should be performed to ensure that all bone fragments and dust are removed before opening the dura. When incising the posterior fossa dura, enough distance from the bony edge is maintained to allow an adequate dural sleeve for a watertight dural closure.
After the dura is reflected, visual inspection of the tumor’s extension to the cortical surface is performed; image guidance or ultrasound may be used if necessary. The surgeon should use multiple anatomic landmarks to confirm navigation data before removing relatively normal-appearing tissue.
Reliance on only one navigation source (CT or MRI computerized navigation) that is prone to error because of brain shift after the dural opening should be avoided. Large surface cortical veins and arteries, normal sulci/gyral pial surfaces on the boundaries of the tumor, and other identifying landmarks on preoperative imaging should be used for complementing surgical orientation during the entire dissection and resection processes.
Details of cortical mapping are discussed in the Language Mapping and Sensorimotor Mapping for glioma chapters. In general, an en bloc tumor resection is preferred if the tumor boundaries or pseudocapsule are relatively distinguishable from the normal brain parenchyma and if there is enough operative space available to achieve this goal without retracting on the normal structures.
The specific techniques for a given tumor are discussed in the individual chapters in this volume. After resection is complete, the surgeon must pay careful attention to achieve hemostasis using bipolar coagulation. I use thrombin solution irrigation for slow venous ooze along the walls of the resection cavity.
I do not cover or line the resection cavity with hemostatic materials because delayed scarring of these materials may lead to their enchantment on postoperative MRIs and confusion regarding tumor recurrence. In addition, these hemostatic materials can convey a false sense of security regarding the level of hemostasis achieved.
Aggressive coagulation of the surrounding normal gyri should be avoided. Persistent bleeding is often because of residual tumor, and further inspection and resection may be required. While a watertight dural closure is necessary after posterior fossa surgery, I do not insist on such a closure for supratentorial craniotomies.
Postoperative Considerations
A neurologic examination should be performed immediately after surgery in the operating room once the patient is awake. If the patient is difficult to arouse or if there is an unexpected focal deficit, a CT scan should be obtained.
Patients can start with ice chips and small sips of water and advance their diet as tolerated. Hourly neurologic checks and vital signs should be performed throughout the first night for select patient who may deteriorate. Many patients can be observed with neurologic evaluations every 2-4 hours.
Typically patients can be transferred out of the intensive care unit or intermediate care unit to the regular ward on the first postoperative day. Antibiotics should be discontinued after 24 hours unless otherwise indicated, and steroids should be continued at a dose of 4mg every 6 hours.
The decision to terminate the use of steroids should be individualized, and patients may be weaned off over 1 to 2 weeks starting on the 4th or 5th postoperative day. Prophylactic anticonvulsant medications are also terminated one week after surgery if the patient has never suffered from a seizure.
An MRI should be obtained within 48 hours of surgery to assess for residual tumor. Physical and occupational therapy should be consulted to aid with any speech, motor, or sensory deficits. Patients may be safely discharged ~3 days after their craniotomy.
References
Apuzzo ML, Chandrasoma PT, Cohen D, Zee CS, Zelman V. Computed imaging stereotaxy: Experience and perspective related to 500 procedures applied to brain masses. Neurosurgery. 1987;20:930-937.
Sawaya R. General principles of brain tumor surgery, in Laligam N, Sekhar RGF (eds): Atlas of Neurosurgical Techniques: Brain, 1st ed. New York: Thieme Medical Publishers, 2006, pp 411-421
Sawaya R, Rambo WM Jr., Hammoud MA, Ligon BL. Advances in surgery for brain tumors. Neurol Clin. 1995;13:757-771.
Tommasino C. Fluids and the neurosurgical patient. Anesthesiol Clin N Am. 2002;20:329-346, vi.
Warner DS, Boehland LA. Effects of iso-osmolal intravenous fluid therapy on post-ischemic brain water content in the rat. Anesthesiology. 1998;68:86-91.
Please login to post a comment.