Epidural Hematoma
Figure 1: Harvey Cushing illustrated an epidural hematoma with an associated skull fracture (circa 1906).
An epidural hematoma (EDH) is one of the most widely known and definitively treatable of all neurologic conditions. In almost all cases, an EDH is caused by blunt trauma leading to a skull fracture. This etiology owes its prevalence to two coinciding phenomena of human evolution. First, the pterion, where the frontal, temporal, sphenoid, and parietal bones meet, is the weakest area of the entire skull and the most likely region to fracture with a traumatic insult.
Second, the middle meningeal artery courses into the skull via the foramen spinosum and proceeds superiorly to the area of the pterion. Thus, the artery and its branches are at risk for direct insult whenever the pterion is fractured.
Figure 2: A large epidural hematoma is formed after a blunt injury to the skull. In most instances, a skull fracture will lacerate the middle meningeal artery and generate a dissecting hematoma. The expanding hematoma will force the dura away from the skull and begin to impart mass effect on the brain and temporal lobe. Uncal transtentorial herniation leads to life-threatening brainstem compression and ischemia.
Presentation
Classically, an EDH occurs after a traumatic blunt blow to the lateral portion of the head provokes a short period of unconsciousness. Upon regaining consciousness, the patient often continues with the previous activity with no regard for the gravity of the situation. The return to previous activity, called the lucid interval, is of variable duration and has been shown to occur in roughly one-third of all patients with EDH.
The expanding hematoma results in declining mental status; the progressive uncal herniation leads to a blown pupil and contralateral hemiparesis. Ultimately, brainstem dysfunction occurs.
Therefore, a failure to diagnose an EDH that is easily treatable can lead to very unfortunate results. The physician must have a high index of suspicion when evaluating any patient who has sustained blunt trauma to the area of the pterion. All health care providers should ensure timely treatment of this life-threatening condition. An accurate history from both the patient and any observers is critical for proper assessment.
Diagnosis
Epidural hematomas typically occur in a patient involved in a head strike (as a result of a blunt injury or even motor vehicle accident) who may or may not have lost consciousness. A computed tomography (CT) scan is required as part of the diagnostic workup. The scan should be ordered without contrast so that any blood in the epidural space can be easily visualized with no loss of pathologic signals caused by contrast-induced noise. In a patient with an EDH, the blood appears bright white on the scan and in the shape of a convex lens. Cranial sutures limit the extent of hematoma because of the attachment of the dura at these locations.
Mass effect may be seen, causing midline shift and ventricular collapse. EDHs are associated with visible skull fractures in 80–90% of patients. Indeed, a CT scan soon after the injury may only depict a skull fracture over the middle meningeal artery, with limited or no blood within the epidural space. In such an instance, an EDH must be completely ruled out with close observation. Depending on the extent of fracture and mechanism of injury, serial CT scans are obtained to exclude an evolving hematoma not visible on the initial imaging.
The classic EDH at the pterion accumulates hematoma within the temporal fossa and is readily visible on imaging. In contrast, a venous EDH caused by a superior sagittal sinus injury expanding at the vertex may be difficult to visualize on an axial CT. A skull fracture at the vertex justifies acquiring coronal images because none of the common indicators, mass effect or ventricular disfigurement, will be readily visualized on an axial CT. If a posterior fossa EDH is suspected, thin-cut CT of the region is mandatory.
Figure 3: An epidural hematoma can occur in multiple locations. Upper row: Note the rapid expansion of a classic right temporal epidural hematoma within a two-hour interval. Lower row: Classic right frontal EDH (left image), right posterior fossa venous EDH (middle image), and a vertex venous EDH (right image). Acute bleeding is an ominous sign and is less hyperdense (swirl sign).
EDHs have been shown to occur concurrently with other traumatic lesions in 25–40% of patients. Associated lesions include brain contusions, coexisting subdural hematomas, and contralateral EDHs.
Indications for Surgery
Indications for surgical or medical management of an EDH are as follows:
- Surgical management
- EDH >30 cm3 regardless of the patient’s Glasgow coma score (GCS)
- Immediate surgical management
- Acute EDH with coma and anisocoria
- Medical (nonoperative) management (controversial)
- EDH <30 cm3 and <15 mm thickness and <5 mm midline shift in a patient with GCS >8 without focal deficit
Nonoperative management includes serial CT scans and close neurologic observation, and must be cautiously considered. The need for prolonged hospitalization for observation and frequent scans during nonoperative approach has encouraged me to consider surgical intervention in most patients with any mass effect from an acute EDH. In addition, any enlargement of the EDH is a reasonable indication for operative evacuation.
There is currently no definitive finding on imaging or clinical exam that can robustly determine whether an acute EDH will continue to enlarge or remain stable. If there is any doubt about whether the patient should proceed to surgery or be medically managed, it is best to proceed with the definitive surgical approach.
Preoperative Considerations
Evaluation of routine coagulation parameters is reasonable if the patient is neurologically stable and not suffering from an impending herniation syndrome. A large stellate scalp laceration with underlying comminuted skull fractures requires the input of a plastic surgeon.
Bilateral EDHs may be challenging to treat. Evacuation of the larger and symptomatic side often leads to enlargement of the contralateral side intra- or postoperatively. Therefore, moderately large bilateral hematomas should be evacuated concurrently. If the smaller one is managed conservatively, serial imaging and prolonged observation is necessary.
SURGICAL EVACUATION OF EPIDURAL HEMATOMA
Once the decision is made to proceed with surgical management, the patient should be quickly prepared for a large craniotomy flap. Special care should be given to both the airway and fluid status in multi-trauma patients.
When planning the incision, it is important to extrapolate the dimensions and borders of the hematoma from the CT images while using the ear as a landmark. The incision must be made large enough so that all of the hematoma and some of the normal dura are exposed. This plan will ensure removal of the coagulated blood; working under the edges of the craniotomy because of a limited bony exposure can be problematic.
The incision and cranial opening must also facilitate adequate exploration of the frontal and temporal lobes, the middle meningeal artery and its branches to the level of the foramen spinosum without significant brain retraction. Injury to the facial nerve is avoided by starting the incision near or superior to the zygoma.
Figure 4: The patient is placed in the supine position and the head is rested on a donut gel pad. A large horseshoe (trauma flap) incision is made in order to expose the dura beyond the edges of the hematoma. As shown, the incision is extended superiorly and posteriorly; the extent of the hematoma behind the auricular helix on the scan determines the posterior limits of the incision. If the patient has suffered a cervical spine fracture, a neck brace is used to keep the neck immobilized while the shoulder is elevated on a large gel pad.
Figure 5: The burr hole and bone flap for EDH evacuation is outlined. In the presence of transtentorial herniation, I expeditiously complete only the lower portion of the incision near the ear and place a large burr hole over the squamous part of the temporal bone so that the hematoma can be accessed and partially drained immediately. I then continue with the rest of the incision.
Upon exposure of the skull, the next step is rapid decompression of the intracranial space. This is performed with a temporal craniectomy by drilling a burr hole. Attempts should be made to evacuate as much of the hematoma as possible through this initial burr hole before proceeding with the rest of the craniotomy.
A full exploration of the epidural space over the frontal and temporal lobes, and the associated middle meningeal artery and its branches to the level of the foramen spinosum is mandatory.
Figure 6: The myocutaneous flap is mobilized. A large burr hole is made posterior to the skull fracture and inferior to the superior temporal line for rapid decompression of the intracranial space. The red dashed outline indicates the boundaries of the bone flap. Skull factures may lead to elevation of a bone flap containing multiple sections. There is no need to expose the lateral aspect of the sphenoid wing. The drill’s footplate may be used to complete the entire osteotomy. Drilling of the pterion is not required.
Figure 7: Once the bone flap is elevated a temporal/subtemporal craniectomy is conducted using a large rongeur. After wide exposure of the hematoma, the clot is manually mobilized and evacuated using irrigation and suction. The hematoma may have expanded since the time of the scan, and meticulous inspection of the surrounding normal epidural space is required. All actively bleeding vessels, including the middle meningeal artery branches, should be coagulated using bipolar electrocautery.
Figure 8: The clot is removed. The bleeding middle meningeal artery is found and coagulated (inset image) along the area of the fracture and in the vicinity of the middle fossa floor. In the event that an obvious bleeding source cannot be found, I follow the route of the middle meningeal artery down to the foramen spinosum and coagulate the artery at its entry site into the intracranial space. I also carefully explore the lateral middle fossa epidural space to remove any additional hematoma underneath the temporal lobe.
In the event that all bleeding sources have been adequately addressed, but there is a small portion of hematoma extending beyond the area of the bone flap, suction and gentle brain retraction may be used to fully evacuate the hematoma. Complete hemostasis is mandatory to prevent a recurrent postoperative hematoma.
After evacuation of the EDH, if the brain is relaxed, I do not consider opening the dura. However, if the injury is severe and the brain is tight upon its palpation transdurally, I create a small slit in the dura and inspect the subdural space for a potential compressive hematoma. Contralateral EDHs, subdural hematomas, and brain contusions can expand immediately upon decompression of the ipsilateral hematoma and lead to brain tension. In this situation, intraoperative ultrasonography can assist with localization of the hematoma. An immediate postoperative CT scan can also identify the underlying reason for lack of brain relaxation and need for further procedures.
Acute Venous Epidural Hematoma
A venous epidural hematoma occurs when the skull fracture overlies a dural sinus such as the superior sagittal or transverse sinuses (see Figure 3 above). The fracture leads to a defect in the wall of the sinus rather than a tear in the middle meningeal artery. This venous bleeding (despite its low pressure) can precipitate large symptomatic mass lesions requiring decompression.
In contrast to my relatively aggressive surgical philosophy for managing arterial EDH, I advocate a conservative approach for handling venous EDHs. Unlike the middle meningeal artery, the dural venous sinuses are indispensable. Elevation of a fractured bone fragment over vital venous sinuses can lead to torrential bleeding from the injured sinus and venous air embolism. Repair and reconstruction of the venous sinus injury is not reliably possible.
Therefore, a venous EDH should be treated nonoperatively unless the resultant mass effect is symptomatic and has led to herniation syndrome or direct brainstem compression (posterior fossa venous EDH). In addition, compared with an arterial EDH, a venous EDH is less likely to expand following the initial injury.
Closure
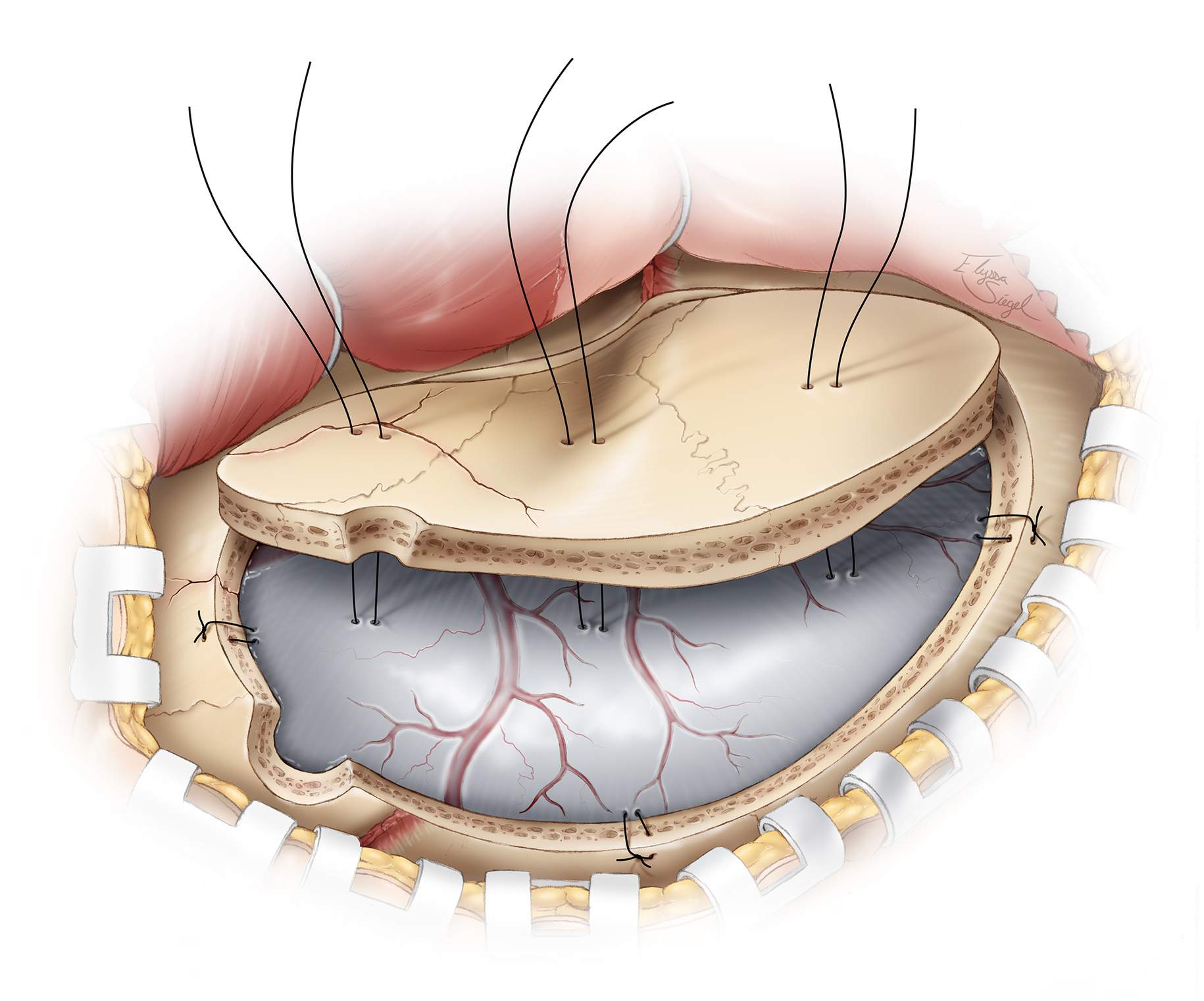
It is important to place numerous tackup stiches along the edges of the craniotomy. I also recommend dural tenting sutures on the bone flap to obliterate the epidural potential space created by the hematoma. This can minimize the risk of hematoma accumulation by any additional pathology that may have been overlooked.
Figure 9: Dural tenting sutures are applied to prevent delayed hemorrhage from dural vessels that are not actively bleeding at the time of closure. The sutures should be placed around and in the center of the bone flap, as shown in this illustration. The sections of the bone flap along the line of skull fracture may be connected using miniplates before its replacement.
An epidural drain may be placed if slow oozing is encountered or if the patient was taking anticoagulants before surgery.
Postoperative Considerations
If the patient cannot be readily awakened or displays new onset focal deficits, a CT scan should be obtained immediately to rule out other compressive lesions, including incomplete hematoma removal, recurrent hematoma, or brain edema. Another scan is performed on the first postoperative day, depending on the surgeon’s preference.
Seizures are a potential complication of any traumatic brain injury and the patient should be prophylactically treated.
Pearls and Pitfalls
- Any patient who has suffered a blunt injury to the skull, especially in the area of the pterion or dural venous sinuses, should be thoroughly evaluated for an EDH. The presence of a skull fracture in the vicinity of these vascular structures demands careful observation and exclusion of an expanding EDH.
- A wide craniotomy and meticulous inspection of the epidural space to expose the source of bleeding is important to relieve the mass effect and prevent hematoma reaccumulation.
Contributor: Jonathan Weyhenmeyer, MD
Please login to post a comment.