Principles of Dural Arteriovenous Fistula Surgery
This is a preview. Check to see if you have access to the full video. Check access
Clip Ligation of a Petrosal/Tentorial Arteriovenous Fistula
Please note the relevant information for patients suffering from dural arteriovenous fistula is presented in another chapter. Please click here for patient-related content.
Intracranial dural arteriovenous fistulas (dAVFs) are a group of acquired pathological vascular malformations that are defined by an abnormal connection between an artery and a vein, bypassing the capillary bed. The shunting that is confined to the dura is supplied by branches of the external carotid artery, tentorial branches of the internal carotid artery, meningeal branches of the vertebral artery, and rarely, the pial branches of the cerebral arteries.
The dAVFs and more specifically the fistulas themselves are located within the walls of the dural venous sinus. They may develop due to dural venous thrombosis, infection, previous surgery, or trauma, although many cases are idiopathic. Inflammatory changes from these conditions can cause angiogenesis, demonstrated by high concentrations of vascular endothelial growth factor (VEGF) found near fistulas. A subset of these fistulas directly connects to a cortical (leptomeningeal) vein.
Some researchers have also proposed embryologic theories that implicate abnormal recanalization of the primitive direct connections between the arteries and veins in response to an inflammatory reaction or venous sinus occlusion.
The frequency of arteriovenous malformations (AVMs) in the general population is approximately 0.15%, and an estimated 10% to 15% of these are dAVFs. Multiple classification systems for dAVFs exist. These systems are based on the lesions’ venous drainage patterns as this factor dictates the behavior of the lesion. Djindjian and Merland first classified dAVFs according to their venous angioarchitecture in 1978. In 1995, Cognard further classified both cranial and spinal arteriovenous fistulas according to their venous outflow with prognostic and treatment implications.
Borden simplified the Cognard classification, emphasizing that the major factor in predicting an aggressive clinical course is the presence of cortical venous drainage. Unlike venous sinuses, cortical veins are not protected by the dura and cannot withstand arterial pressures. Therefore, dAVFs with cortical venous drainage (Borden types II and III) have a higher risk of rupture and hemorrhage. The hemorrhage from dAVFs can be parenchymal, subarachnoid, or subdural in nature.
| Borden Classification | Cognard Classification |
| Type I: Flow into dural venous sinus or meningeal vein |
Type I: Anterograde drainage into a dural venous sinus Type IIa: Retrograde drainage into a dural venous sinus |
| Type II: Flow into a dural venous sinus + CVR |
Type IIb: Anterograde drainage into a dural venous sinus + CVR Type IIa + b: Retrograde drainage into a dural venous sinus + CVR |
| Type III: CVR only |
Type III: CVR only without venous ectasia Type IV: CVR only with venous ectasia Type V: Spinal perimedullary venous drainage |
CVR: Cortical venous reflux also referred to as cortical venous drainage and retrograde leptomeningeal venous drainage.
Intracranial hemorrhage and neurologic deficit is likely in 2% of the Borden classification type I, 39% of type II, and 79% of type III dAVFs.
The most common presentation of a low-grade (Borden Type I) dAVF in patients is pulsatile tinnitus, which may be auscultated as a bruit by the clinician. Other presentations include headaches or deteriorating mentation from the effects of venous congestion. Hydrocephalus or edema may occur as a result of an obstructive outflow in a large venous varix or from impaired cerebrospinal fluid (CSF) drainage caused by increased venous sinus pressures.
The natural history of dAVFs without cortical venous drainage is fairly benign; only 1% of these lesions convert from Borden types I and II to Borden type III. However, there is a 45% mortality rate over 4 years among patients with cortical venous drainage, a 19.2% intracranial hemorrhage rate per year and 10.9% new neurologic deficit rate per year. For patients who present with hemorrhage, the rehemorrhage rate is 35% within the first 2 weeks of the initial ictus. Venous stenosis is a concerning sign and suggests the risk of transformation to a more malignant one or the loss of venous access.
The surgical approach to many dAVFs may be associated with significant blood loss, and endovascular therapy is therefore the treatment of choice for most intracranial locations, with a few notable exceptions, such as the ethmoidal and petrosal/tentorial dAVFs. These operative dAVFs are associated with higher risks of hemorrhage than fistulas in other sites. I will review the subtypes of intracranial dAVFs before discussing their surgical management.
Classifications and Operative Considerations
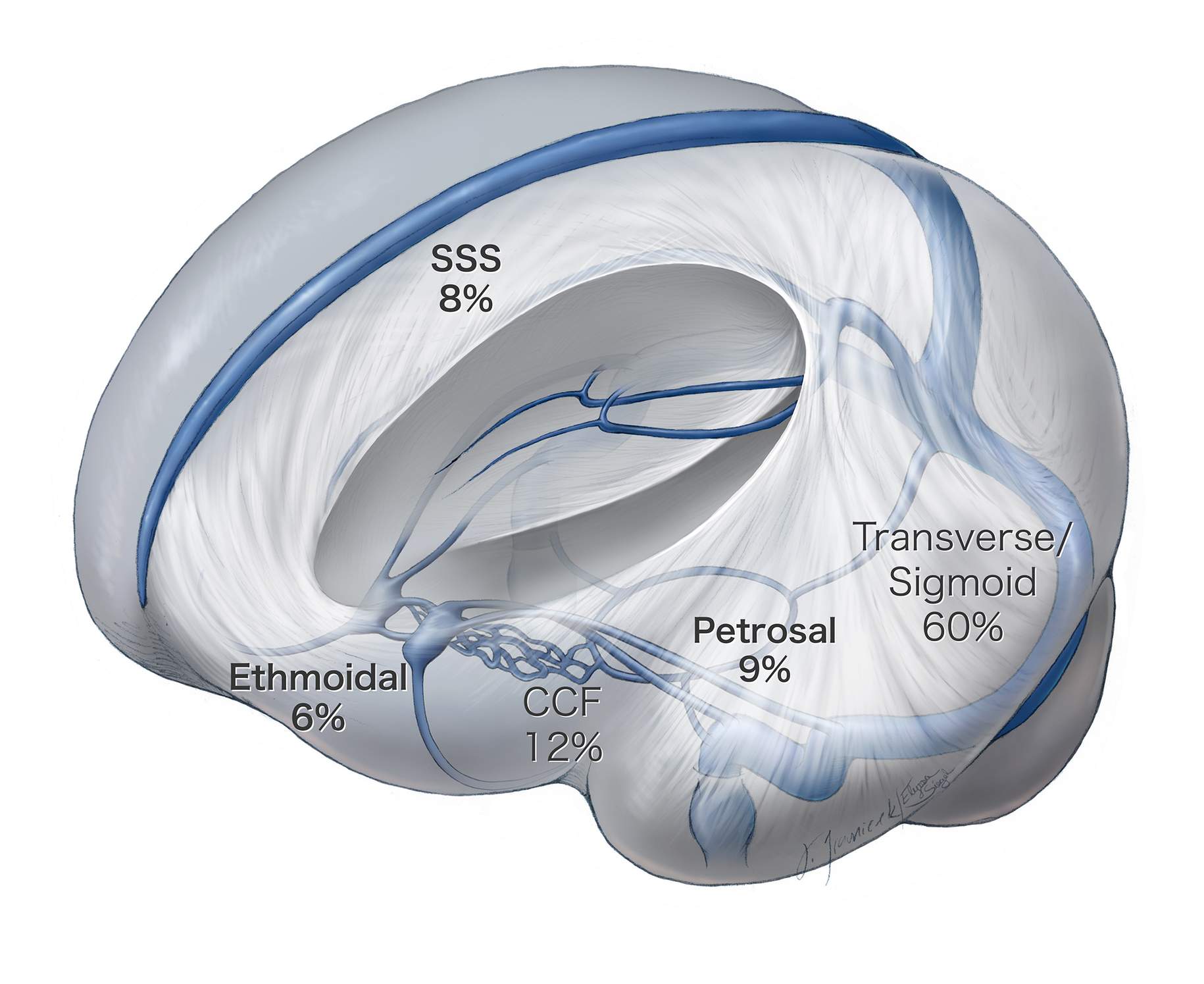
The majority (60%) of intracranial dAVFs are found along the transverse-sigmoid junction, followed by the cavernous sinus and the superior sagittal sinus. The lesions of the transverse and sigmoid junction are largely treated endovascularly because there is a direct transvenous route that allows for occlusion of the fistula or the venous sinus.
Figure 1: This illustration demonstrates the location and incidence of the most common types of intracranial dAVFs. Ethmoidal and petrosal fistulas are most favorable for microsurgical ligation, unlike other dAVFs, which are more amenable to endovascular therapy.
The venous sinuses may be occluded through embolization as long as there is an alternate collateral venous drainage route. For instance, in the case of a left transverse-sigmoid dAVF, a patent torcula allows for occlusion of the left transverse sinus (as long as the vein of Labbé is spared). Similarly, the cavernous sinus often must be completely embolized to cure a carotid cavernous fistula (CCF), and venous drainage is rerouted to the sphenoparietal sinuses and the Sylvian veins that join the superior sagittal sinus.
Transarterial embolization may be used to reach the venous side of the fistula. However, it is necessary to embolize the venous side because occlusion of the arteries alone will result in recanalization of the fistula via the smaller fistulous feeders, not initially detectable or amenable to embolization.
Diagnosis and Evaluation
Computed tomography (CT) or magnetic resonance imaging (MRI) may demonstrate diffusely engorged venous congestion of the draining veins or the presence of a dilated superior ophthalmic vein in a patient with a cavernous-carotid fistula. The characteristic angiographic feature of dAVFs is premature appearance of intracranial veins or venous sinuses during the arterial phase.
Magnetic resonance angiography (MRA) or CT angiography (CTA) may not detect a dAVF. Three-dimensional MRA may be useful, but traditional catheter or digital subtraction angiography remains the gold standard for the diagnosis and management of dAVFs. The angiogram should evaluate both internal carotid arteries, both external carotid arteries, and both vertebral arteries. A thorough angiogram is mandatory because internal carotid or vertebral artery injections alone may lead the physician to overlook a large dAVF fed by the external carotid circulation. In addition, even a simple dAVF may recruit multiple feeding arteries from different circulations of there could be multiple dAVFs.
The nidus of a dAVF is the center of arteriovenous shunting and the site of the dura where all feeding arteries converge to and the venous draining channels diverge from. Although multiple draining veins and intimidating engorged varices and veins are apparent, a single large draining vein is most often the main draining site of the fistula.
The presence or absence of cortical venous drainage, venous sinus occlusion, direction of flow (anterograde versus retrograde) in the dural venous sinuses, and the normal venous drainage anatomy of the surrounding cortices must be evaluated.
I carefully study the venous phase of the angiogram to ensure that normal brain is not draining into an indispensable vein (vein of Labbe) that is joining the arterialized target vein. If that is the case, the nonarterialized vein(s) should be carefully spared during the disconnection of the arterialized ones.
Supratentorial Dural Arteriovenous Fistulas
The majority of superatentrial fistulas that are amenable to operative intervention are ethmoidal and parasagittal dAVFs.
Figure 2: An anteroposterior angiogram of the left external carotid artery (left) and a lateral angiogram of the left internal carotid artery (right) demonstrate an ethmoidal dAVF that is fed by the anterior ethmoidal and falcine arteries and drains into an arterialized cortical vein associated with venous varices.
Ethmoidal fistulas are located at the anterior fossa floor and fed by the anterior ethmoidal arteries, the dural branches of the ophthalmic artery, and the anterior falcine artery that arises from the ophthalmic artery. They may also recruit dural arterial supply via the anterior division of the middle meningeal arteries. They serve a fistulous connection harboring pial vein(s)(olfactory and frontal veins) that connect at the base of the anterior fossa dura just under the frontal lobe or medially into the falx.
Venous varices on the arterialized vein carry a significant risk of hemorrhage, shown to be up to 57% in some series. Due to the pial nature of these veins, there is no practical transvenous route to reach them. The transarterial route is through the ophthalmic artery, rendering transarterial embolization a risk for blindness. Surgical treatment, however, is technically easy and low risk and is described in the chapter titled: Supratentorial Dural Arteriovenous Fistulas.
Infratentorial Dural Arteriovenous Fistulas
The majority of infratentorial dAVFs that are suitable to microsurgery are superior petrosal dAVFs.
Figure 3: A lateral internal carotid artery (ICA) angiogram demonstrates a tentorial/petrosal dAVF, supplied by the tentorial feeders from the ICA and draining into the petrosal vein with arterialization of the posterior fossa veins.
Superior petrosal dAVFs are also difficult to access endovascularly from either the arterial or venous side. They feed from the tentorial branches of the internal carotid artery, such as the tentorial artery of Bernasconi and Casinari, the inferolateral trunk, and the meningohypophyseal trunk, as well as the external carotid branches, such as the middle meningeal and ascending pharyngeal arteries. They drain into the arterialized petrosal vein and produce large supratentorial or infratentorial arterialized varices. These lesions are usually easily treated via clip ligation of the arterialized superior petrosal sinus through the retrosigmoid approach. This technique is described in the chapter titled Infratentorial Dural Arteriovenous Fistulas.
A large venous varix associated with the fistula can cause trigeminal neuralgia by compression of the root entry zone of the trigeminal nerve.
Cavernous-Carotid Fistulas
One unique type of intracranial dAVF is the cavernous-carotid fistula (CCF), a connection between the carotid artery and the cavernous sinus. This connection may be direct from the cavernous carotid artery itself, which is usually high-flow and due to trauma, or indirect from the arterial feeders from the internal or external carotid artery.
CCFs are unique in their presenting ophthalmic symptoms, although they can also present with symptoms of retrograde cortical venous drainage. Initial symptoms include a proptotic, chemotic eye with increased intraocular pressure (IOP). Glaucoma (IOP > 20) can lead to blindness, and is considered an urgent condition requiring treatment.
CCFs may also present with third, fourth, or sixth cranial nerve palsies and are best treated through endovascular embolization of the cavernous sinus accessed via the inferior petrosal sinus, superior ophthalmic vein, or more rarely, the basilar plexus. A direct puncture of the superior ophthalmic vein or the cavernous sinus via pterional craniotomy is occasionally required for access.
Indications for Microsurgery
As shown by the natural history, untreated dAVFs with cortical venous drainage carry a high risk of morbidity and mortality. The dAVF must be treated with occlusion of the venous side of the fistula; arterial occlusion alone frequently will not result in an effective and durable cure.
Lesions that do not harbor cortical venous drainage do not require treatment unless they are associated with intolerable tinnitus, visual deterioration and/or pain. For these lesions, the aim of treatment is palliative and not curative.
Unlike arteriovenous malformations (AVMs), which have a nidus within the parenchyma and are subject to hemorrhage and rupture in case of venous occlusion before all of their arterial feeders are disconnected, the dAVF’s nidus (or fistula) is within the contained thickened leaves of the dura. Therefore, venous occlusion is safe and curative.
Three microsurgical strategies are available for dAVFs. One strategy provides venous access for direct embolization/packing of the pathologic dural venous sinus (pterional craniotomy and cavernous sinus puncture for CCFs). Another strategy involves resection of the dAVF and associated pathologic dural leaflets and venous sinuses. The third and most applicable and employed one is sole disconnection of the arterialized leptomeningeal draining vein(s), without lesional excision.
Certain dAVFs without a transarterial or transvenous access route (without any sinusal drainage) are addressed microsurgically. Examples of operative fistulas are ethmoidal, or anterior fossa, and superior petrosal sinus, or tentorial, fistulas; both of which almost always demonstrate cortical venous drainage since they are not related to a dominant venous sinus. Disconnection of the arterialized vein(s) is all that is needed to prevent the future risk of hemorrhage.
MICROSURGICAL DISCONNECTION OF dAVFs
For a discussion of microsurgical ligation of supratentorial and infratentorial dAVFs, please refer to their corresponding chapters.
For patients who present with an acute hemorrhage related to their dAVF, I do not consider repair of the fistula an emergent or urgent procedure unless there is an evidence of symptomatic mass effect from the hemorrhage. If emergent evacuation of the hematoma is not required, I plan to proceed with obliteration of the fistula during the next available elective operative day available on my schedule (within 2-3 days). Urgent intervention, as indicated for ruptured aneurysms, does not apply.
Alternative Approaches
Endovascular therapy is now the primary mode of treatment for most dAVFs. Transvenous embolization is the primary approach, although transarterial embolization is being used more frequently in the era of Onyx, because Onyx can often be pushed through the fistula to the venous side if the catheter can be navigated close to the nidus.
Transarterial embolization can spare the sinus if the catheter can be navigated into the fistula that is contained within the wall of the sinus, using a transvenous balloon to protect the lumen of the sinus during liquid embolic embolization.
Stereotactic radiosurgery may be used for certain fistulas or components of fistulas that cannot be accessed by endovascular or surgical means. There have been some reports of cure using gamma knife procedures; however, more studies are necessary to determine the efficacy of radiotherapy in these high-flow dural-based lesions. In addition, the long interval between application of radiation and therapeutic effect may place patients at risk due to 15-20% per year risk of hemorrhage.
Microsurgical Resection of dAVFs
Resection of dAVFs involves disconnection of the arterial feeders and arterialized cortical veins as well as circumferential excision of the pathologic dural leaflet(s) and associated occluded/nonfunctional dural venous sinus. The involved dural venous sinus is resected if it is not responsible for the venous drainage of normal brain. I recommend preoperative transarterial embolization of the arterial feeders to reduce intraoperative blood loss that can be quite voluminous. Despite these measures, torrential hemorrhage is likely during scalp incision and bone work.
The craniotomy or -ectomy may have to often be done by drilling the bone in layers due to the presence of numerous transosseous feeders that require ample amount of bone wax for their control. The affected dural leaflets and involved venous sinuses are generously exposed on all their sides. Next, the involved dura and feeding arteries are aggressively cauterized, clipped and devascularized. If the involved venous sinus can be safely sacrificed, the dura is incised circumferentially on both sides of, and parallel to the venous sinus. Ultimately, the involved segment of the venous sinus is ligated proximally and distally and excised along with the pathologic dura.
All the arterialized cortical draining veins are severed at their entrance into the venous sinus. I routinely use intraoperative angiography to ensure complete obliteration of the fistula. If the venous sinus is functional and involved in normal venous drainage of the brain, it should be skeletonized and left in situ.
Thorough skeletonization of the venous sinus requires complete interruption of the dural arterial supply to the fistulous segment of the sinus followed by disconnection of the dural leaflets around all sides of the sinus. This maneuver allows preservation of the venous sinus and the nonarterialized cortical veins. Transverse/sigmoid or posterior superior sagittal sinus dAVFs require coagulation and disconnection of the tentorium and the falx adjacent to the sinus. Pathologic arterialized vein(s) can be distinguished from normal nonarterialized vein(s) using intraoperative fluorescence angiography.
Transverse/Sigmoid Sinus dAVF
The dAVFs of the transverse/sigmoid sinuses are the most common intracranial dAVFs. Their main arterial supply arises from the transmastoid branches of the occipital artery, the posterior auricular artery, the middle meningeal artery, and potentially the ascending pharyngeal artery. Venous drainage is via the ipsilateral transverse/sigmoid sinuses or through the contralateral side if the ipsilateral sinus is occluded.
These lesions are best managed via endovascular therapy. Rarely, this modality is not effective or endovascular access is limited and microsurgery is undertaken. Preoperative transarterial embolization is crucial in decreasing the risk of intraoperative bleeding.
I prefer the park-bench position, head turned toward the floor, and the lesion placed at the highest point on the head. A horseshoe or “S”-shaped incision is used to generously expose the area of the fistula as guided by intraoperative image guidance based on a CT angiogram.
The hypertrophied occipital and posterior auricular arteries are coagulated or clipped and divided. The scalp and suboccipital muscles carry numerous feeders to the fistula and stepwise immaculate hemostasis is paramount.
The craniotomy exposes the transverse sinus and the dura above and below it. I usually prefer to perform a craniectomy rather than a craniotomy to prevent the sudden torrential bleeding from the transosseus feeders to the dura during elevation of the bone flap. Any dural laceration is avoided via additional burr holes. Excessive bleeding from the dura is controlled via tamponade using large pieces of Gelfoam or Surgicel. Dural feeders are managed via bipolar electrocautery or hemostatic clips.
The intradural portion of the operation is conducted based on the specific anatomy of the underlying dAVF. If disconnection of the arterialized cortical vein(s) is the main goal, the dura is incised at the corresponding location of these veins and based on the transverse sinus. All arterialized vein should be found and disconnected. Intraoperative fluorescence and catheter angiograms can help confirm the exclusion of the target(s).
If the goal of surgery is resection of the fistulous segment of the occluded sinus, a unilateral partial mastoidectomy is completed using a diamond burr mounted a high-speed drill. This maneuver should expose the dura lateral and anterior to the sigmoid sinus. The dura is incised superior and inferior to the transverse/sigmoid sinuses and parallel to their long axes. Feeding arteries are coagulated or clipped in a stepwise fashion. Following skeletonization of the nonfunctional transverse/sigmoid sinus segments, I place two sutures through the tentorium to ligate the target segment of the sinus.
I gently elevate the occipital lobe and retract the cerebellum after gradual lumbar CSF drainage. This maneuver allows me to expose the tentorium further and disconnect all its feeding vessels to the ligated section of the sinus and cut the tentorium parallel to this section. This technique releases the isolated pathologic segment of the dural sinus.
All arterialized leptomeningeal veins are also found and divided. The drainage site of the nonarterialized vein of Labbe into the transverse or sigmoid sinus should be protected. If the vein of Labbe is arterialized, it should be coagulated and cut.
Other Considerations
For patients who are not suffering from cortical venous drainage, observation is reasonable. I repeat an angiogram every 3-5 years to assess the dynamic angioarchitecture of the malformation and exclude the risk of newly developed cortical venous drainage.
Pearls and Pitfalls
- Occlusion of the venous side of the fistulous connection must occur for cure of dAVF.
- Endovascular therapy is the first-line treatment for most types of dAVF, including cavernous-carotid fistulas and those near the transverse and sigmoid sinuses, and superior sagittal sinus, because surgical therapy can be associated with a significant blood loss.
- However, ethmoidal and petrosal dAVFs continue to remain amenable to microsurgical intervention because of the challenges associated with endovascular reach to the target veins and the low risk of microsurgery.
Contributors: Thomas Wilson, BS, and Stacey Quitero-Wolfe, MD
References
Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671-680.
Davies MA, Ter Brugge K, Willinsky R, Wallace MC. The natural history and management of intracranial dural arteriovenous fistulae. Part 2: aggressive lesions. Interv Neuroradiol. 1997;20:303-311.
Gross BA, Du R. Surgical treatment of high grade dural arteriovenous fistulae. J Clin Neurosci. 2013;20:1527-1532.
Hwang G, Kang HS, Oh CW, Kwon OK. Surgical obliteration in superior petrosal sinus dural arteriovenous fistula. J Korean Neurosurg Soc. 2011;49:222-225.
Liu JK, Dogan A, Ellegala DB, Carlson J, Nesbit GM, Barnwell SL, Delashaw JB. The role of surgery for high-grade intracranial dural arteriovenous fistulas: Importance of obliteration of venous outflow. J Neurosurg. 2009;110:913-920.
Lucas CP, Zabramski JM, Spetzler RF, Jacobowitz R. Treatment for intracranial dural arteriovenous malformations: a meta-analysis from the English language literature. Neurosurgery. 1997;40:1119-1130; discussion 1130-1132.
Javadpour M, Wallace MC. (2012) Surgical Management of Cranial Dural Arteriovenous Fistulas in A Quinones-Hinojosa (Eds.), Schmidek and Sweet’s Operative Neurosurgical Techniques, Saunders, Philadelphia, PA.
Miller NR. Dural carotid-cavernous fistulas: Epidemiology, clinical presentation, and management. Neurosurg Clin N Am. 2012;23:179-192.
Sarma D, ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. (Review). Eur J Radiol. 2003;46:206-220.
Winn RH. Treatment of other intracranial dural arteriovenous fistulas, in: Youman’s Neurological Surgery. Philadelphia: Saunders, 2011.
Please login to post a comment.