Anteromedial Temporal Lobectomy
This is a preview. Check to see if you have access to the full video. Check access
Technical Principles of Temporal Lobectomy and Amygdalohippocampectomy
The estimated prevalence of epilepsy in North America is 5 to 10 cases per 1000 people, and approximately one-third of those with epilepsy fail to achieve adequate seizure control with antiepileptic drugs or are unable to tolerate the side effects required to do so. A large body of evidence indicates that failure to adequately control seizures impairs cognition, decreases overall quality of life, and increases mortality.
Medical intervention is the first step in treating epilepsy. Despite the use of various antiepileptic drugs, when medical therapy fails to achieve seizure freedom, comprehensive surgical evaluation is appropriate. The most common and well-characterized focal epilepsy syndrome is medial (mesial) temporal lobe epilepsy (MTLE). For patients with intractable MTLE, an anterior temporal lobectomy and amygdalohippocampectomy (ATL) offers the possibility of seizure freedom with reduced reliance on antiepileptic medications.
The ATL has been refined over the past century from its earliest descriptions by Falconer and Morris into a safe and effective procedure that has been validated with class I evidence. This procedure has consistently provided seizure-free rates approaching 70% or better in MTLE patients. This chapter outlines the modalities used for diagnosis and evaluation of temporal lobe epilepsy (TLE), my surgical approach to MTLE, and outcomes after surgery.
Diagnosis and Evaluation of Temporal Lobe Epilepsy
From an electrical and clinical perspective, TLE can be divided into two different subtypes: MTLE and neocortical temporal lobe epilepsy (NTLE). The distinction between these subtypes is made based on differences with respect to electrophysiology, neuropsychological profile, pathophysiology, and response to surgery.
The most common cause for TLE is medial (mesial) temporal sclerosis (MTS). Pathologically, this is characterized by segmental loss of pyramidal cells, dispersion of granule cells, and reactive gliosis. Other causes of TLE include tumors, infections, vascular malformations, cortical dysplasia, and trauma. The differential diagnosis for NTLE is similar to MTLE, with the exception of MTS.
Among patients with epilepsy caused by an extratemporal epileptogenic focus or lesion, approximately 15% have associated MTS; these patients harbor what is termed dual pathology. It is not clear which lesion (or sometimes both) serves as the true epileptogenic focus in these patients; resection of both foci generally yields the highest likelihood of attaining seizure freedom as long as preoperative evaluation demonstrates localization of the seizure focus to one or both of the two sites. The kindling phenomenon has been implicated in causation of MTS in this patient population. Despite resection of the extratemporal focus, MTS can continue to act as an autonomous source of seizure generation.
The goal of epilepsy surgery is removal of the epileptogenic zone, which, if completely resected, potentially results in seizure freedom. The preoperative evaluation of TLE seeks to identify this zone and determine the safety of resective surgery for the seizure focus. Herein I will review the various diagnostic modalities involved with the diagnosis of the epileptogenic zone in TLE.
History and Physical Examination
The preoperative evaluation includes a detailed history and physical exam. Seizure semiology, past medical history, family history, remote history of febrile seizures/trauma/meningoencephalitis and details regarding attempted antiepileptic medications should be discussed. Often it is helpful if a family member or friend is present who has witnessed typical or habitual seizure episodes. A complete neurologic examination, in conjunction with the history, may help estimate the location of the seizure focus.
The presentation of patients with TLE often follows a characteristic electroclinical syndrome that includes an abdominal sensation (aura) followed by hand/mouth automatisms and possible ictal contralateral dystonic posturing of the upper extremity. Postictal speech dysfunction indicates lateralization of seizure onset to the dominant hemisphere. Unfortunately, patients with MTLE and NTLE have similar clinical presentations and cannot be distinguished from one another based on clinical features alone. As expected, NTLE is associated with more frequent generalized seizures.
Ambulatory and Sleep-Deprived Electroencephalogram
Scalp electroencephalogram (EEG) is an essential component of the initial evaluation and is usually performed on an outpatient basis for convenience. A 30-minute awake/sleep-deprived analysis may help localize the epileptogenic focus by correlation of the ictal and interictal discharges with clinical history and imaging findings.
Many patients with MTLE harbor unilateral anterior temporal interictal spikes on surface EEG. Additionally, some investigators report unilateral temporal rhythmic theta activity less than 30 seconds after electrical seizure onset to be associated with ipsilateral MTLE. The sensitivity of detecting an EEG abnormality can be increased by performing the test within 48 hours of a known seizure.
Video Electroencephalography
A critical component in determination of surgical candidacy requires inpatient admission to the epilepsy monitoring unit for continuous scalp EEG and video monitoring. Correlation of the clinical seizure manifestations with interictal and ictal discharges helps localize the epileptogenic focus. Provocative measures, such as medication reduction, sleep deprivation, and hyperventilation, may be used to induce epileptiform activity. If scalp EEG fails to lateralize the epilepsy focus, invasive EEG monitoring using intracranial electrodes (an intracranial study) may be necessary to provide further localization.
Invasive EEG Monitoring (Intracranial Study)
Scalp EEG fails to lateralize the seizure focus in up to one-third of patients, and even when noninvasive tests are lateralizing, up to 10% are falsely localizing. Therefore, invasive EEG monitoring (an intracranial study) should be undertaken when there is discordance among the different preoperative tests, suspicion of multifocal epilepsy, or MRI-negative TLE that requires differentiating NTLE from MTLE. This intracranial study will delineate the borders of the epileptogenic focus and its proximity to the eloquent cortices.
The characteristic electrical activity from the recording hippocampal depth electrodes for MTLE includes periodic spiking activity from the hippocampus, followed by episodes of high-voltage rhythms lasting up to 1 minute. As the adjacent entorhinal cortex and temporal neocortex are recruited, a synchronous and regular 5 to 9 Hz ictal rhythm is often seen in the subtemporal and anterior scalp EEG electrodes.
Another common EEG pattern for MTLE includes low-voltage, high-frequency discharge without preictal spiking. This activity is recorded by the hippocampal depth electrodes and medial-basal subdural electrodes in contact with the entorhinal cortex. In NTLE, the most common ictal onset is characterized by low voltage and high-frequency discharge that is associated with loss of background activity.
After scalp/invasive video EEG monitoring, patients who are found to suffer from psychogenic nonepileptic seizures, multifocal epilepsy, a generalized seizure disorder, or a nonlocalizing seizure focus are deemed not appropriate candidates for resective surgery. Almost half of the patients who undergo scalp EEG and video monitoring harbor a well-defined epilepsy focus or require an intracranial study to determine their surgical candidacy.
Imaging
Magnetic resonance (MR) imaging is the primary diagnostic imaging modality of choice for TLE. The MR protocol should scan the whole head, thin-sectioned high-resolution T1- and T2-weighted images, in addition to gradient echo T2 sequence to investigate the presence of blood.
If a mass lesion or tumor is found, gadolinium should be administered to better establish the character of the lesion and its anatomic location. If MTLE is suspected, the required sequences include coronal T1-weighted imaging, fluid-attenuated inversion recovery (FLAIR) coronal sequences, and T2-weighted coronal sequences through the hippocampus.
Typical findings of mesial temporal sclerosis are hippocampal atrophy and increased FLAIR and T2 signal hyperintensity in the medial temporal lobe structures. Hippocampal atrophy on preoperative MR imaging is associated with improved seizure control following temporal lobectomy. MR imaging, in combination with the other diagnostic modalities discussed in this chapter, affords a more accurate localization of the epileptogenic focus.
Figure 1: Left medial temporal lobe sclerosis is demonstrated. Note the left hippocampal atrophy and subtle hyperintensity on T2 and FLAIR sequences.
Neuropsycological Assessment
Neuropsycological evaluation is important for patients with TLE because it can identify preoperative functional deficits and predict postoperative neuropsychological outcomes. Planned surgical removal of the temporal lobe and hippocampus necessitates the evaluation of language and memory, respectively. Patients with dominant lobe TLE display subtle verbal memory deficits and word-finding difficulty. In contrast, patients with nondominant TLE typically display visuospatial memory dysfunction.
The most common postoperative deficit following TLE surgery is memory decline. Verbal memory deficits are more likely to occur after surgery on the left temporal lobe, compared with visuospatial deficits following surgery on the right side. Patients undergoing a left temporal lobectomy who have average or better memory and language function are at higher risk for developing postoperative deficits. However, no such relationship has been found in patients who undergo right temporal lobectomy. A preoperative discussion with the patients at risk for postoperative memory or language dysfunction must be conducted before surgical treatment is offered.
Wada Test
Before the advent of functional MRI (fMRI), the Wada test was used to assess language and memory function of the two cerebral hemispheres independently. It is now used when fMRI is inconclusive, not available, or not achievable. Amobarbital or another anesthetic agent, such as methohexital, propofol, or etomidate, is injected into the internal carotid artery, temporarily suppressing function on that side while language and memory tests are performed. Baseline memory function is typically tested the day before the actual test.
Before injection of the anesthetic agent, the patient is asked to raise both arms and count aloud. Hemiplegia signifies adequate anesthesia. Language and memory are assessed while hemiplegia persists, evaluating the side harboring the suspected epilepsy focus first. Next, the contralateral hemisphere is tested 30 minutes later.
Language representation is considered to be unilateral (right or left) if global aphasia occurs after anesthetic injection into one hemisphere, and bilateral (with or without predominance) if some degree of preservation of language is seen after injection in both hemispheres.
For memory evaluation, patients are asked to correctly identify items shown during the hemiparesis phase. An overall memory score is assigned based on the ability of the contralateral side to support memory during anesthetic injection of the side ipsilateral to the suspected epilepsy focus. Memory scores of 50% to 67% have been deemed passing scores. The goal of the memory portion of the Wada test is to establish risk for postoperative memory decline. The less memory support the contralateral hemisphere owns, the greater the risk for postoperative memory deficit.
Despite the high accuracy of the Wada test in determining language and memory dominance, this test is still associated with false positives and negatives. Additionally, Wada test results can be affected by drug dose, unblinding of test assessors, and patient cooperation. Furthermore, the Wada test is associated with risks such as seizures, contrast allergy, catheter site hematoma, vascular dissection, stroke, and infection. The risk of arterial dissection or stroke is estimated at 1%.
Positron Emission Tomography
Positron emission tomography (PET) utilizes radioactive isotopes linked to metabolically active molecules to analyze functionality in various regions of the body based on metabolic activity. When investigating TLE, PET seeks to localize the regions of interictal hypometabolism in the temporal lobe.
EEG recording during PET is important to ensure that hypometabolism in one hemisphere is not secondary to an active seizure on the contralateral side resulting in hypermetabolism.
Fluorodeoxyglucose (FDG) is the most commonly used isotope in PET. FDG-PET has a high specificity for MTLE. Mesial temporal sclerosis is associated with hypometabolism within the hippocampus, amygdala, entorhinal cortex, and temporal pole.
It has also been shown that hypometabolic regions identified using FDG-PET correlate well with the predicted laterality when compared with the results of depth electrodes. The sensitivity of PET is increased when the metabolic activity from both temporal lobes is sampled to examine hypometabolism in one temporal lobe as compared with the other.
PET does not provide any additional information if MTS is found on MRI. Therefore, PET is typically reserved for cases in which MRI and other preoperative tests fail to localize the seizure focus.
Single Photon Emission Computed Tomography
Cerebral blood flow to the brain is increased in regions of the brain undergoing seizure activity due to increased metabolic demand. Single photon emission computed tomography (SPECT) measures local cerebral perfusion using technetium-99m hexamethyl propelene amine oxime or technetium-99m bicisate. These compounds are taken up by neurons within seconds of injection and remain in the cells for several hours. Therefore, injecting radiotracers immediately following a seizure may help localize the area of ictal onset.
The sensitivity of SPECT is increased if interictal studies are used to compare the relative change in cerebral perfusion during seizure activity. Additionally, the difference in blood flow between interictal and ictal SPECT can be correlated and coregistered with high-resolution MRI to identify the epilepsy focus. SPECT is typically reserved for cases in which MRI and/or EEG are nonlocalizing.
Functional Magnetic Resonance Imaging
Functional MRI (fMRI) examines neuronal activity via alterations of the MR signal due to changes in blood oxygenation levels. It is mainly used for localization of the eloquent cortex, such as motor and language cortices. It can also be coupled with EEG analysis to identify the zone of ictal onset. It has a spatial resolution of a few millimeters, and can be used as a noninvasive alternative to the Wada test for language lateralization and localization of the cortical speech areas.
As a newer imaging modality, only a handful of studies have correlated fMRI and surgical outcome. Increased signal activation during language and memory tasks ipsilateral to the ictal focus has been associated with greater deficits after surgical resection, a correlation that may be a stronger predictor than neuropsychological testing.
Indications for Surgery
Patients with medically intractable epilepsy of medial temporal lobe origin, as defined by failure of two or more antiepileptic medication trials, are candidates for anteromedial temporal resection and amygdalohippocampectomy.
Patients may undergo resective surgery without invasive intracranial monitoring if they have: 1) ictal onset lateralized to one hemisphere on EEG, 2) unilateral MTS on MRI and/or evidence of unilateral mesial disease on PET or SPECT, 3) neuropsycological and Wada/fMRI tests to confirm memory and speech dysfunction consistent with the side of ictal onset, and 4) Wada confirmation that the contralateral side supports memory function. Patients who do not meet these criteria may need an intracranial study using electrodes and further testing with the aforementioned modalities before a surgical resection is performed.
The need for an intracranial study is dictated based on a suspicion raised by EEG or imaging modalities that epileptogenesis is not limited to the medial temporal lobe. The absence of hippocampal atrophy and presence of a neocortical dysplastic structural lesion on imaging studies, as well as documentation of neocortical ictal onset by basic invasive electrode recordings, indicate the need for a more in-depth intracranial study with adequate coverage of candidate cortices by subdural grid electrodes for ictal localization and language mapping. The use of depth electrodes within the medial structures is routine.
Right-handed individuals do not require a Wada study for right temporal lobe resections due the high likelihood of the left temporal lobe housing verbal memory.
Preoperative Considerations
In the above sections, I discussed the details of preoperative comprehensive epilepsy surgery evaluation for TLE. The use of intraoperative image guidance using MR imaging is advised for the novice surgeon. This tool guides the entrance into the temporal horn via the middle temporal gyrus.
The perioperative events and anesthetic medications lead to a drop in the serum levels of anticonvulsant medications, exposing the patient to the risks of postoperative seizures. Supratherapeutic levels of anticonvulsant medications are therefore advised during the perioperative period because seizures complicate the patient’s recovery from surgery.
I do not use intraoperative electrocorticography to guide the extent of neocortical or hippocampal resection. If the extent of seizure focus is in question, intracranial monitoring is more effective to guide the surgeon.
Operative Anatomy
The anatomy of the medial temporal lobe is certainly the most complex part of the intraparenchymal anatomy.
Click here to view the interactive module and related content for this image.
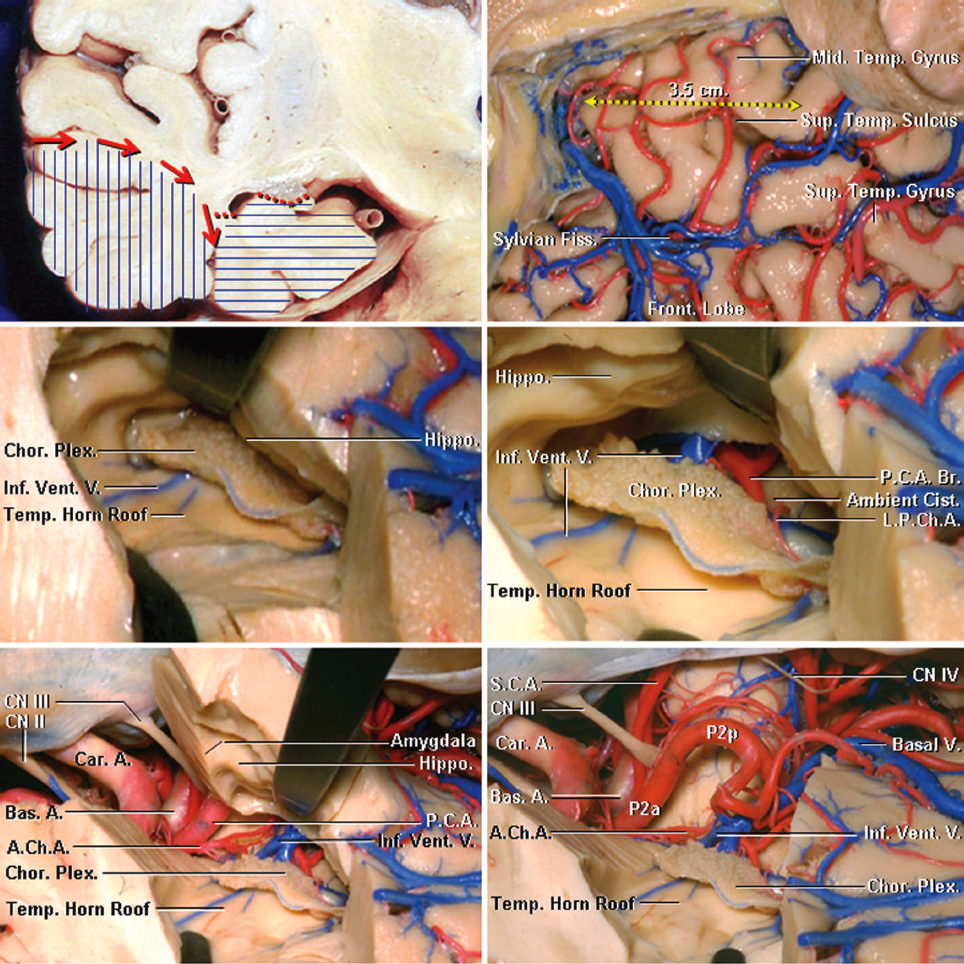
Figure 2: The superficial anatomy of the temporal lobe is shown (upper rows of images). The inferior choroidal point is defined as the entry point of the anterior choroidal artery into the temporal horn approximated by the anterior edge of the choroid plexus. A line connecting the inferior choroidal point to the middle cerebral artery bifurcation approximates the border between the amygdala and striatum (lower photos)(images courtesy of AL Rhoton, Jr). The proximity of the amygdala to the striatum is evident.
Click here to view the interactive module and related content for this image.
Figure 3: The surface cortical anatomy is reviewed. The fusiform or occipitotemporal gyrus is separated laterally from the inferior temporal gyrus by the occipitotemporal sulcus at the basal temporal lobe surface, while medially it is separated from the parahippocampal gyrus by the collateral posteriorly and the rhinal sulci anteriorly. The borders of the anterior, middle, and posterior medial temporal lobe are marked by brackets. The anterior portion of the lobe consists of the uncus (green bracket). The middle portion ends at the level of the quadrigeminal plate (red bracket), while the posterior segment ends at the calcarine point (blue bracket). Note the coronal anatomy of the medial temporal lobe (lower rows)(images courtesy of AL Rhoton, Jr).
Figure 4: The anatomy of the temporal horn from a superior perspective is shown. Note the demarcation of the medial temporal lobe from this perspective into the anterior, middle, and posterior compartments (left image). The vascular anatomy from a similar perspective is also shown (right image). Note the location of the anterior choroidal artery (AChA) and its entrance into the ventricle via the inferior choroidal point.
Click here to view the interactive module and related content for this image.
Figure 5: The steps involved in performance of a right-sided anteromedial temporal neocortical resection and amygdalohippocampectomy are shown. Note the coronal presentation of the neocortical white matter dissection (red arrows) beginning from the superior or middle temporal gyri toward the collateral sulcus. Neocortical resection (vertical blue lines) usually spares the superior temporal gyrus. Resection of the amygdala, uncus, hippocampus, and parahippocampal gyrus is performed as part of the second phase of the operation (horizontal blue lines). The neocortex spanning 3.5 cm from the temporal tip involving the inferior and middle temporal gyri are removed (right upper image). The temporal horn has been uncapped to expose the choroid plexus and hippocampus (middle left image). The choroidal fissure has been opened, and the structures within the ambient cistern have been exposed by separating the choroid plexus from the fimbria of the fornix (right middle image). The choroid plexus should remain attached to the thalamus. The vascular anatomy of the medial temporal lobe along the basal temporal lobe and ventricular surface is demonstrated (lower row). The posterior cerebral artery (PCA) is the main arterial feeder to the medial temporal lobe. The PCA gives rise to the anterior inferior temporal artery, from which the anterior parahippocampal artery arises. The anterior choroidal artery enters the temporal horn at the inferior choroidal point that is found at the posterosuperior edge of the uncus. A line connecting the inferior choroidal point to the middle cerebral artery bifurcation approximates the border between the amygdala and striatum and is a reasonable operative landmark.
ANTEROMEDIAL TEMPORAL LOBECTOMY AND SELECTIVE AMYGDALOHIPPOCAMPECTOMY
The details of technique for a standard ATL involve a very defined set of steps which prevent operator’s disorientation that can lead to injury to the thalamus and the contents of the basal cisterns.
Figure 6: The patient is positioned supine with the head turned 50 degrees contralaterally and stabilized via a skull clamp. A shoulder roll may be used to assist with lateral head rotation. The single pin is placed over the mastoid area to keep the arms of the skull clamp out of the operator’s working zone.
Figure 7: The patient’s head is markedly extended toward the ipsilateral shoulder to flatten the long axis of the hippocampus. This head position is imperative because it provides the surgeon direct visualization through the temporal horn and along the axis of the hippocampus during intraventricular dissection and medial hippocampal disconnection. A reverse question mark incision is used for exposure (shown in blue). The incision begins near the zygoma and extends posteriorly behind the ear just in front of the mastoid vertex line, then curves anteriorly just above the insertion line of the temporalis muscle (superior temporal line). I mobilize the temporalis muscle flap to identify the root of zygoma. It is important to remember that this craniotomy aims to predominantly expose the temporal lobe and not the frontal lobe. The outline of the craniotomy is marked.
Figure 8: A myocutaneous flap is elevated and a single burr hole is placed at the root of the zygoma or just below the superior temporal line at the posterior aspect of the exposure. A Penfield #3 dissecting instrument mobilizes the dura. A craniotomy is performed as illustrated. An anterior temporal craniectomy exposes the most anterior and inferior aspects of the temporal fossa so that I can direct my microscope’s view relatively parallel to the axis of the hippocampus during the later stages of dissection. A curvilinear dural incision affords reflection of the dural flap anteriorly so that the temporal tip is nearly reachable.
Further details about the performance of the exposure and craniotomy are summarized in the Pterional Craniotomy chapter.
INTRADURAL PROCEDURE
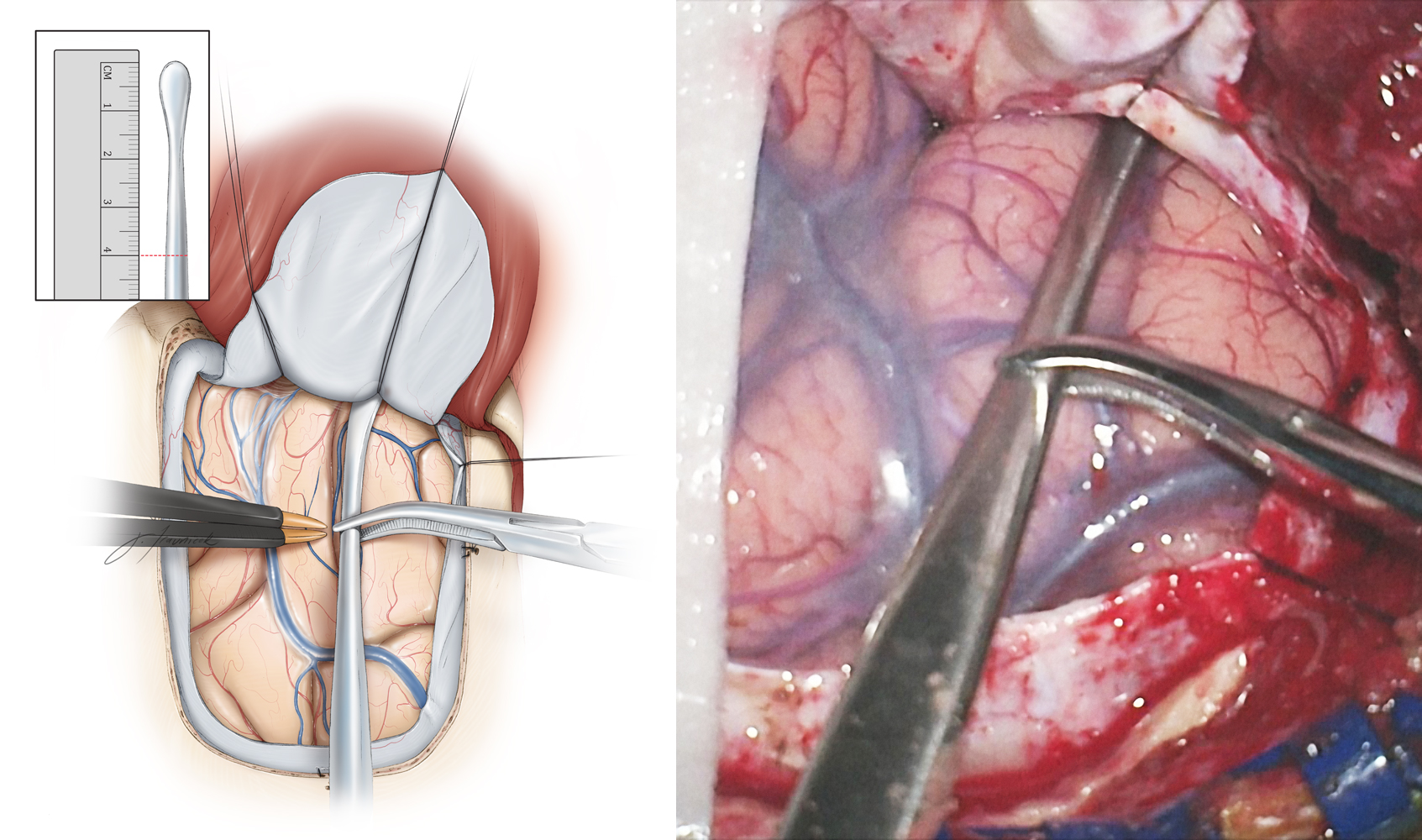
Figure 9: I use a #1 Penfield dissecting instrument to determine the extent of the lobectomy measured from the temporal tip. I mark the 4.0-cm line on the instrument (inset image) and then slide the instrument over the middle temporal gyrus until the tip of the instrument touches the anterior temporal fossa just below the sphenoid wing. The extent of the lobectomy from the temporal tip is marked on the cortex with an attempt to preserve the middle temporal veins located anterior to the vein of Labbe. The extent of neocortical resection is approximately 4 cm and 3.5 cm for the nondominant and dominant temporal lobes, respectively.
Figure 10: The superior corticotomy is carried out along the superior temporal sulcus. Preservation of the superior temporal gyrus, which may contain language function in the dominant temporal lobe, is advised. Next, the posterior corticotomy is performed perpendicular to the long axis of the middle and inferior temporal gyri, 3.5 to 4 cm from the temporal pole. The depth of resection is about 2 to 3 cm and includes the fusiform gyrus. As the operator gains additional experience, the fusiform gyrus resection may be extended more medially to the level of the ventricle, simplifying exposure of the temporal horn and mobilization of the mesial structures. Intraoperative navigation can guide this step of the operation.
Bridging veins from the Sylvian fissure to the sphenoparietal sinus are identified at the anterior temporal tip. Retraction of the temporal lobe can cause bleeding from these veins unless they are cauterized during this portion of the procedure.
Figure 11: After the superior and posterior corticotomies, white matter dissection is performed to disconnect the lateral temporal neocortex. There is an unnamed but anatomically consistent bony protuberance (yellow arrow) along the middle fossa floor that can be found via gentle retraction of the temporal lobe. This protuberance is a useful landmark for guiding white matter dissection. The planes of the superior and posterior corticotomies and white matter dissections should join at this bony protuberance. Dissection along these two planes defined by the corticotomies and the bony protuberance will ensure that the operator will not violate the temporal horn prematurely or injure the vital structures located medial to this plane of dissection.
Figure 12: The underlying location of the temporal horn in relation to the deep white matter of the middle temporal gyrus is indicated. The temporal horn is the key landmark for the exposure and resection of medial structures. The temporal horn lies deep to the white matter previously covered by the middle temporal gyrus. Entry into the ventricle (at the tip of bipolar forceps) is confirmed by the appearance of cerebrospinal fluid and identification of the choroid plexus. Upon entering the ventricle, I incise the lateral wall of the ventricle to expose the medial structures.
One should recognize that the ventricle is more posterior than expected due to the size of the amygdala that occupies the anteromedial temporal lobe. If the operator is disoriented during subcortical dissection, he or she may continue medial white matter dissection and completely miss the ventricle and inadvertently enter the brainstem, thalamus, or structures in the center of the hemisphere.
Figure 13: Exposure of the hippocampus is expanded by disconnection of the occipitotemporal fasciculus on the lateral wall of the ventricle. This dissection is parallel to the lateral edge of the hippocampus. Moreover, the fusiform gyrus is incised along the occipitotemporal fasciculus until the arachnoid of the mesial occipitotemporal gyrus is identified. The choroid plexus is apparent at the tip of the suction device (lower intraoperative photo).
The Meyer’s loop courses along the roof and lateral aspects of the temporal horn. The operator can avoid a superior temporal quadrantanopsia by maintaining the plane of dissection within the medial fusiform gyrus.
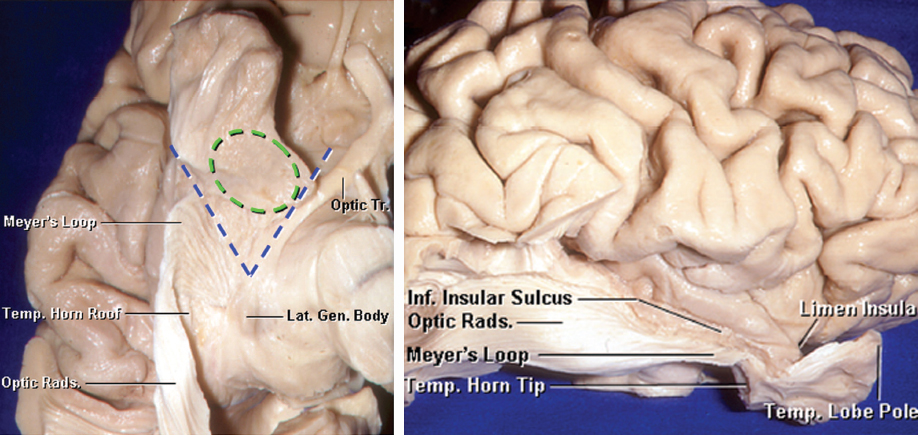
Figure 14: An inferior view of the right temporal lobe is demonstrated (left image). The gray and white matter underneath the temporal horn and optic radiations have been removed. The optic radiations emerge from the lateral geniculate body, and loop forward on the ventricular roof toward the anterior temporal horn. These radiations subsequently turn posteriorly around the roof and lateral wall of the temporal horn and atrium. A section of the amygdala (outlined in green) has been included. The blue lines mark the triangular area between the Meyer’s loop and the optic tract with the apex at the lateral geniculate body, through which the temporal horn can be accessed via the floor of the Sylvian fissure without interrupting the optic radiations or optic tract. A lateral view in also included (right image)(images courtesy of AL Rhoton, Jr).
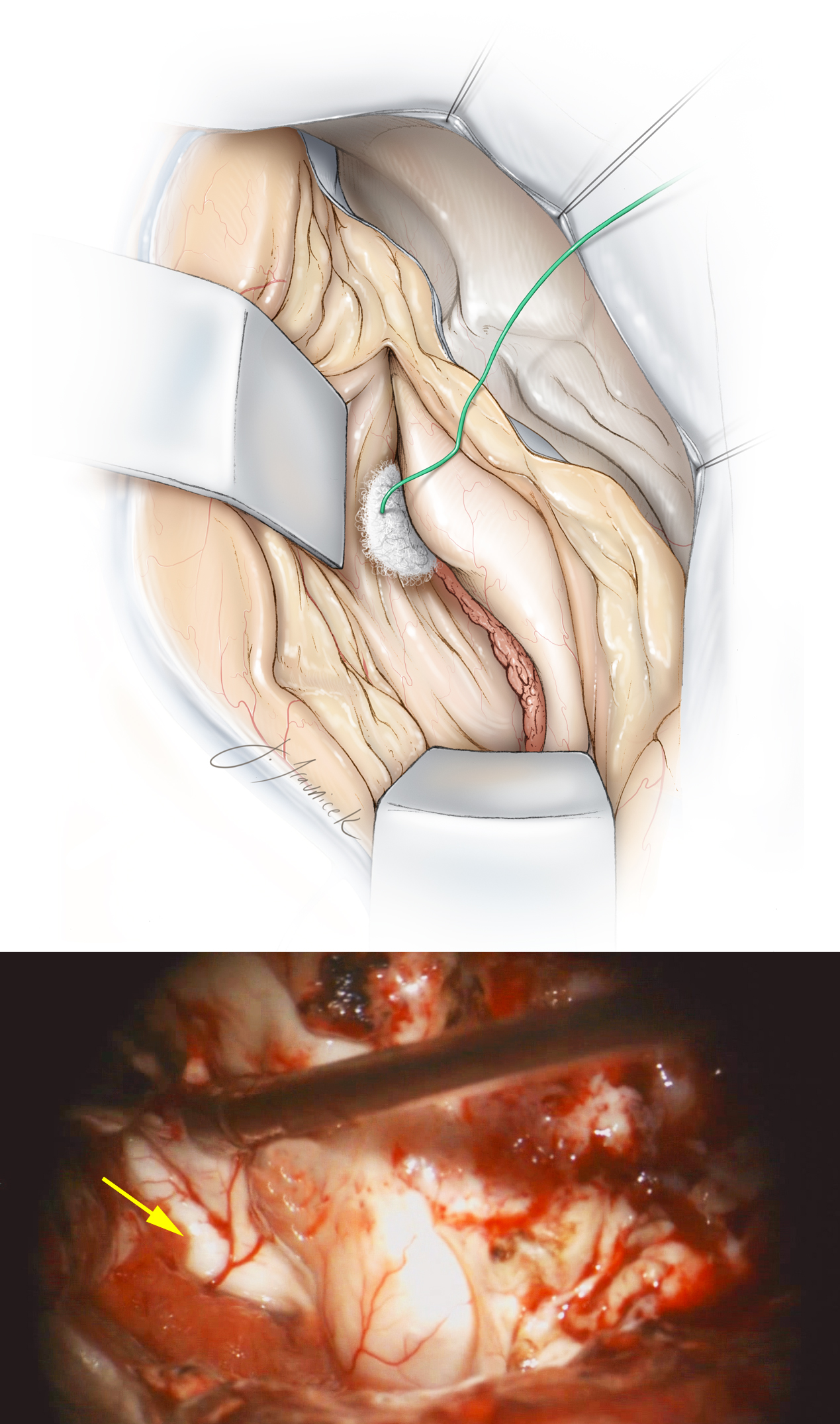
Figure 15: My colleagues prefer to use two fixed retractor blades, one to support the superior temporal gyrus, and the second on the cut surface of the posterior plane of resection. One retractor elevates the roof of the temporal horn and allows identification of the choroid plexus and hippocampal structures. The second retractor blade mobilizes the remainder of the temporal neocortex laterally. A small cottonoid pledget is placed into the temporal horn and at the anterior edge of the choroid plexus so that the inferior choroidal point is easily identified. Another pledget is placed at the tail of the temporal horn to prevent blood from entering the rest of the ventricular system. The yellow arrow identifies the uncinate fasciculus and the intralimbic lobe of the parahippocampus just underneath the choroid plexus.
Figure 16: A small portion of the medial middle temporal gyrus overlying the middle cerebral artery (MCA) is subpially removed to expose the MCA bifurcation or the distal M1 segment that remains covered by its encasing Sylvian fissure arachnoid layers. A line from the MCA to the inferior choroidal point (where the anterior choroidal artery enters the temporal horn) defines the border (green plane) between the amygdala and the pallidum. It is important to maintain this dorsal dissection plane during the amygdalectomy to avoid injuring the globus pallidus (top illustration). The bottom intraoperative photo shows the M1 through it arachnoid band (red arrow) and the inferior choroidal point (blue arrow). The connecting black line marks the upper border of amygdalar removal.
Figure 17: The inferior two-thirds of the amygdala and the entire uncus of the parahippocampus are highlighted in white and green, respectively. Resection of the mesial structures begins by subpial evacuation of these structures. Removal of the amygdala is conducted utilizing the dorsal plane of dissection mentioned previously.
Figure 18: An en bloc amygdalectomy is feasible while maintaining the ventral plane of dissection limited to the arachnoid layers covering the basal cisterns. I maintain the integrity of these arachnoid layers during the entire process of medial dissection to protect the underlying oculomotor nerve, PCA, and brainstem. Identification of the oculomotor nerve and the free edge of the tentorium through their corresponding arachnoid membranes confirms adequate medial removal of the uncus (upper inset image). The posterior limit of the dissection is where the uncus joins the head of the hippocampus (lower inset image). I use a flat dissector to peel the remaining thin layer of the uncus off of the overlying arachnoid of the optic nerve (intraoperative photo).
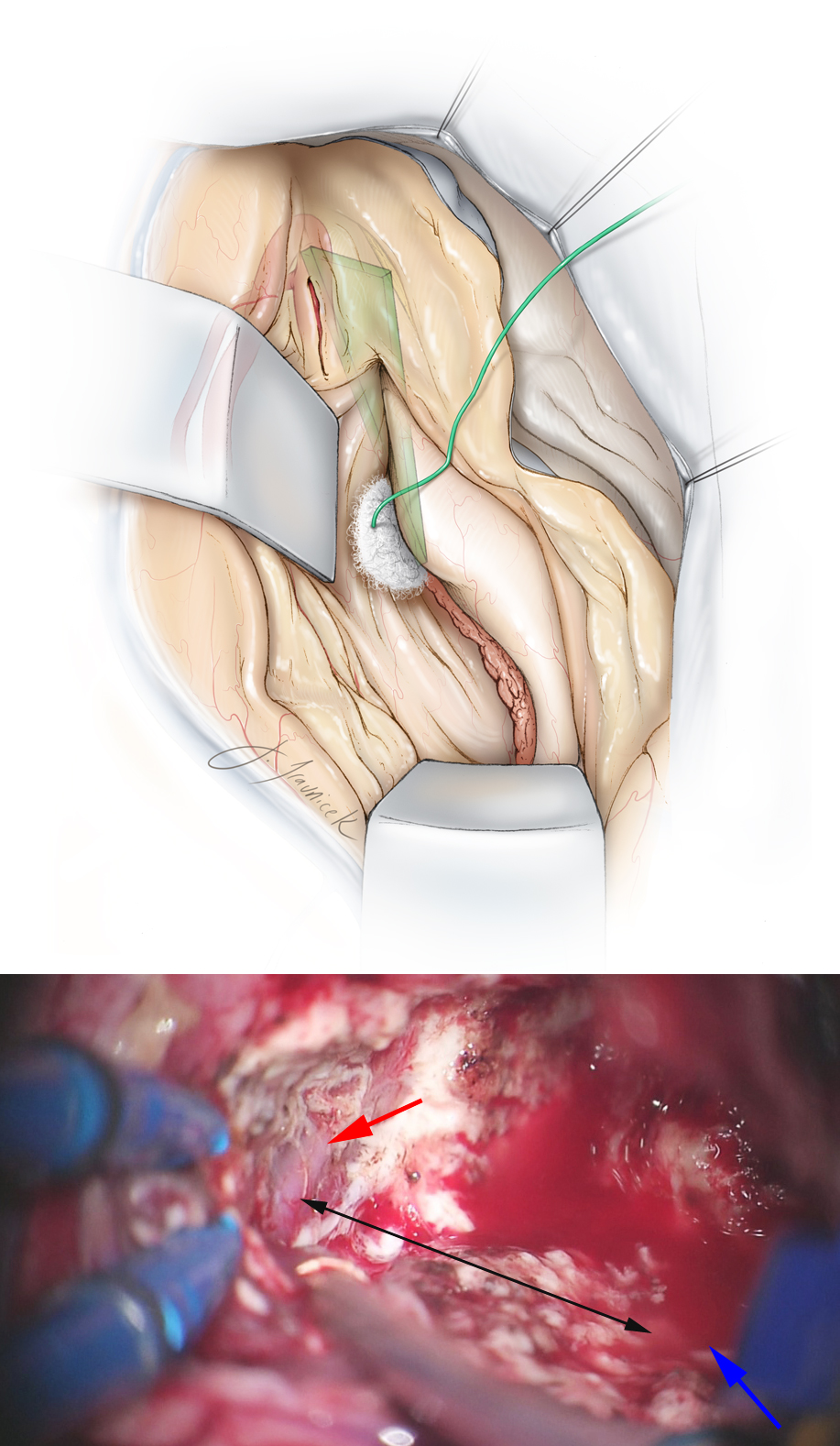
Figure 19: After removal of the amygdala and uncus, the dissection through the medial hippocampus proceeds posteriorly through the uncinate fasciculus and the intralimbic lobe of the parahippocampus toward the hippocampus proper. The disconnection of the medial part of the hippocampus remains lateral to the choroid plexus to avoid inadvertent injury to the anterior choroidal artery (a cause of hemiplegia from ATL operations). The hippocampus is covered by a small cotton patty during its manipulation by the suction device.
Figure 20: The arachnoid bands underneath the parahippocampal gyrus are sectioned. The pes hippocampus (pes) is then separated from the body of the hippocampus near the inferior choroidal point. This maneuver allows subpial elevation and anterior mobilization of the pes and parahippocampal gyrus as one unit.
Figure 21: After extraction of the pes, the posterior parahippocampal gyrus is mobilized away from the collateral sulcus until the posterior portion/tail of the hippocampus is seen to curve medially toward the calcar avis. The arteriolar branches of the posterior cerebral artery to the hippocampal body will be encountered during dissection of the collateral sulcus and anterior mobilization of the hippocampal body/tail. These small arteries are cauterized once they are well within the sulcus and severed sharply to avoid avulsion injury to the parent vessel and other vessels coursing near this space. The combined block of posterior parahippocampal gyrus and hippocampus are elevated and removed. Dissection over the hippocampal fissure and arachnoid bands over the brainstem and thalamus allows mobilization of the medial parahippocampal gyrus, hippocampus, and fimbria (top illustration). The middle photo demonstrates the PCA (blue arrow) and perforating arterioles to the posterior hippocampus (yellow arrow). Care must be taken to maintain the arachnoid layers over the brainstem and perforating vessels to avoid postoperative neurologic deficit (bottom photo; the red arrow points at the top edge of the PCA).
Figure 22: The remaining tail of the hippocampus before it turns medially within the ventricle is subpially evacuated via the use of the hand-held suction device or by transecting the hippocampus across its tail. I use the hand-held suction device to dynamically mobilize the posterior neocortex laterally and create access to the posterior portions of the hippocampus. A thin layer of adherent brain tissue may be left on the arachnoid layers covering the basal cisterns to minimize their risk of tear. Aggressive coagulation along the edge of the tentorium is avoided to minimize the risk of injuring the trochlear nerve.
The extent of hippocampal resection is associated with improved postoperative seizure outcome. Hippocampectomy to the level of the quadrigeminal plate is advised. Retained mesial structures is the most common cause of recurrent seizures.
Anteromedial Temporal Lobectomy and Selective Amygdalohippocampectomy
Additional Considerations
If there is evidence of MTS associated with an adjacent temporal epileptogenic lesion, dual pathology is suspected. I remove the lesion and the nondominant hippocampus to maximize the chance of postoperative seizure freedom. However, if the lesion does not directly affect the dominant hippocampus, the dominant hippocampus should be spared to minimize the risk of postoperative memory dysfunction.
Postoperative Considerations
Supratherapeutic levels of anticonvulsant medications are advised during the perioperative period. Seizures during the immediate postoperative period are not uncommon and do not indicate a poor long-term seizure outcome from surgery. The patient and his or her family should be reassured.
Anticonvulsant drugs are continued for approximately 1 to 2 years postoperatively, even in the absence of seizures during this period. If the patient remains seizure free during this time, cessation of these medications may then be considered.
Pearls and Pitfalls
- Early identification of the temporal horn orients the operator anatomically and prevents misguided operative trajectories.
- Limiting the extent of occipitotemporal fasciculus dissection along the lateral hippocampus minimizes the breadth of expected visual field defects caused by injury to the Meyer’s loop.
- Transient neuropathies of cranial nerves III and IV can be avoided by meticulous protection of the arachnoid planes and by avoiding bipolar cauterization near the tentorial edge.
- Compromise of the anterior choroidal artery can be avoided by minimizing bipolar cauterization and manipulation of the choroid plexus. Cottonoid patties cover the choroid plexus to avoid its direct manipulation which leads to bleeding.
- The posterior cerebral artery can remain safe during hippocampus mobilization by carefully cauterizing and transecting the perforating vessels entering the hippocampal sulcus once they are well within the fissure.
Contributor: Stephen Mendenhall, MD
References
Cendes F, Cook MJ, Watson C, Andermann F, Fish DR, Shorvon SD, et al. Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology. 1995;45:2058-2064.
Chelune GJ, Naugle RI, Luders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991;41:399-404.
Chou CC, Shih YH, Yen DJ, Kwan SY, Yu HY. Long-term health-related quality of life in drug-resistant temporal lobe epilepsy after anterior temporal lobectomy. Epileptic Disord. 2015; 17:177-183.
Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346:140-144.
Dodrill CB, Ojemann GA. An exploratory comparison of three methods of memory assessment with the intracarotid amobarbital procedure. Brain Cogn. 1997; 33:210-223.
Duncan JS. Imaging and epilepsy. Brain 1997;120(Pt 2):339-377.
Engel J, Jr., Kuhl DE, Phelps ME, Crandall PH. Comparative localization of epileptic foci in partial epilepsy by PCT and EEG. Ann Neurol. 1982;12:529-537.
Engel J, Jr., Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60.538-547.
Falconer MA, Meyer A, Hill D, Mitchell W, Pond DA. Treatment of temporal-lobe epilepsy by temporal lobectomy; a survey of findings and results. Lancet. 1955;268:827-835.
Gaillard WD, Bhatia S, Bookheimer SY, Fazilat S, Sato S, Theodore WH. FDG-PET and volumetric MRI in the evaluation of patients with partial epilepsy. Neurology. 1995; 45:123-126.
Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425-432.
Hoppe M, Wennberg R, Tai P, Pohlmann-Eden B. Textbook of Stereotactic and Functional Neurosurgery, ed 2nd. Berlin, Germany: Springer, 2009.
Janszky J, Jokeit H, Kontopoulou K, Mertens M, Ebner A, Pohlmann-Eden B, et al. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia. 2005; 46:244-250.
Jokeit H, Schacher M. Neuropsychological aspects of type of epilepsy and etiological factors in adults. Epilepsy Behav. 2004(Suppl 1):S14-20.
Jones-Gotman M. Neuropsychological techniques in the identification of epileptic foci. Epilepsy Res. 1992; (Suppl 5):87-94.
Jung WY, Pacia SV, Devinsky R. Neocortical temporal lobe epilepsy: intracranial EEG features and surgical outcome. J Clin Neurophysiol. 1999;16:419-425.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314-319.
Lee SK, Kim JY, Hong KS, Nam HW, Park SH, Chung CK. The clinical usefulness of ictal surface EEG in neocortical epilepsy. Epilepsia. 2000; 41:1450-1455.
Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43:283-291.
Li LM, Cendes F, Andermann F, Watson C, Fish DR, Cook MJ, et al. Surgical outcome in patients with epilepsy and dual pathology. Brain. 1999;122( Pt 5):799-805.
Loddenkemper T, Morris HH, Moddel G. Complications during the Wada test. Epilepsy Behav. 2008;13:551-553.
Malow BA, Blaxton TA, Sato S, Bookheimer SY, Kufta CV, Figlozzi CM, et al. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37:245-252.
Mansouri A, Fallah A, Valiante TA. Determining surgical candidacy in temporal lobe epilepsy. Epilepsy Res Treat. 2012:706917.
McIntosh AM, Wilson SJ, Berkovic SF. Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia. 2001;42:1288-1307.
Medina LS, Bernal B, Ruiz J. Role of functional MR in determining language dominance in epilepsy and nonepilepsy populations: a Bayesian analysis. Radiology. 2007;242:94-100.
Mirsattari SM, Ives JR, Leung LS, Menon RS. EEG monitoring during functional MRI in animal models. Epilepsia. 2007;48(Suppl 4):37-46.
Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13:277-282.
Morris AA. Temporal lobectomy with removal of uncus, hippocampus, and amygdala; results for psychomotor epilepsy three to nine years after operation. AMA Arch Neurol Psychiatry. 1956;76:479-496.
O'Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445-454.
Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868-9872.
Pacia SV, Ebersole JS. Intracranial EEG in temporal lobe epilepsy. J Clin Neurophysiol. 1999;16:399-407.
Passarelli V, Pinto LF, Jorge CL, Puglia P, Adda CC, Wen HT, et al. The intracarotid etomidate Wada test: a 54-patient series. Epilepsy Behav. 2014;39:73-77.
Rausch R, Babb TL, Engel J Jr, Crandall PH. Memory following intracarotid amobarbital injection contralateral to hippocampal damage. Arch Neurol. 1989;46:783-788.
Roessler K, Sommer B, Grummich P, Coras R, Kasper BS, Hamer HM, et al. Improved resection in lesional temporal lobe epilepsy surgery using neuronavigation and intraoperative MR imaging: favourable long term surgical and seizure outcome in 88 consecutive cases. Seizure. 2014;23:201-207.
Ryvlin P, Bouvard S, Le Bars D, De Lamerie G, Gregoire MC, Kahane P, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain. 1998; 121 ( Pt 11):2067-2081.
Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL 3rd, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788-1792.
Simkins-Bullock J. Beyond speech lateralization: a review of the variability, reliability, and validity of the intracarotid amobarbital procedure and its nonlanguage uses in epilepsy surgery candidates. Neuropsychol Rev. 2000;10:41-74.
Spanaki MV, Spencer SS, Corsi M, MacMullan J, Seibyl J, Zubal IG. Sensitivity and specificity of quantitative difference SPECT analysis in seizure localization. J Nucl Med. 1999;40:730-736.
Spencer SS. When should temporal-lobe epilepsy be treated surgically? Lancet Neurol. 2002;1:375-382.
Spencer SS, Williamson PD, Bridgers SL, Mattson RH, Cicchetti DV, Spencer DD. Reliability and accuracy of localization by scalp ictal EEG. Neurology. 1985;35:1567-1575.
Sperling MR, O'Connor MJ, Saykin AJ, Phillips CA, Morrell MJ, Bridgman PA, et al. A noninvasive protocol for anterior temporal lobectomy. Neurology. 1992;42:416-422.
Walczak TS, Leppik IE, D'Amelio M, Rarick J, So E, Ahman P, et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology. 2001;56:519-525.
Westerhuis W, Zijlmans M, Fischer K, van Andel J, Leijten FS. Coping style and quality of life in patients with epilepsy: a cross-sectional study. J Neurol. 2011;258:37-43.
Wiebe S, Bellhouse DR, Fallahay C, Eliasziw M. Burden of epilepsy: the Ontario Health Survey. Can J Neurol Sci. 1999;26:263-270.
Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness, Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311-318.
Yildirim Capraz I, Kurt G, Akdemir O, Hirfanoglu T, Oner Y, Sengezer T, et al. Surgical outcome in patients with MRI-negative, PET-positive temporal lobe epilepsy. Seizure. 2015;29:63-68.
Please login to post a comment.