Pituitary Microadenoma
Figure 1: Harvey Cushing’s career in neurosurgery was initially founded on the surgical management of pituitary tumors. In the absence of adequate imaging, functional adenomas were more likely to be diagnosed and surgically explored. In my review of ~15,000 patient photographs in the Cushing Brain Tumor Registry, these are the only two photos in which Cushing himself is included. The patient on the left was diagnosed with acromegaly and the one on the right may have suffered from progeria.
Please note the relevant information for patients suffering from pituitary tumors is presented in another chapter. Please click here for patient-related content.
This is a preview. Check to see if you have access to the full video. Check access
Acromegaly: Principles of Microadenomectomy
Diagnosis and Evaluation
Pituitary tumors are diverse—they range from nonfunctional to functional and from millimeters to centimeters. The majority of functional adenomas overproduce one or more of the pituitary hormones, which may be prolactin, growth hormone, adrenocorticotropin, or thyroid stimulating hormone. See the chapter on Pituitary Adenoma: Diagnosis and Operative Considerations for a more in depth discussion of evaluation for these specific tumors.
Indications for Surgery
Prolactinomas and adrenocorticotropic hormone (ACTH)-secreting adenomas are the most common form of microadenomas.
Prolactinomas may undergo surgical resection if they fail to regress with dopaminergic therapy or if the patient suffers adverse effects from medications. If pituitary apoplexy causes symptomatic chiasmal compression, urgent surgical decompression is necessary. Furthermore, cystic prolactinomas may not respond as effectively to medical therapy and may require cyst decompression. Tumors that cause extensive skull base erosion may also undergo surgery because tumor regression via medical treatment may induce a cerebrospinal fluid leak.
ACTH-secreting adenomas are most often microadenomas and are diagnosed relatively early. The standard of care is operative intervention. Because these microadenomas are typically centrally located within the gland and can be more fibrous and infiltrative, their intraoperative localization and resection can be difficult.
Refer to the chapter on Pituitary Adenoma: Diagnosis and Operative Considerations for more details about surgical indications.
Preoperative Considerations
If there is ambiguous radiologic evidence regarding the ACTH-secreting adenoma’s presence or location, petrosal sinus sampling can help centralize and lateralize a hypersecreting tumor. Identification of the side that is likely hyperfunctional can guide resection of 40% of the gland on the corresponding side in an attempt to remove a microadenoma that is not readily identifiable intraoperatively or diffusely affecting the gland on that side.
For a complete discussion of the standard preoperative considerations for patients with a pituitary adenoma, see the chapter on Pituitary Adenoma: Diagnosis and Operative Considerations.
TRANSNASAL TRANSSPHENOIDAL RESECTION OF PITUITARY MICROADENOMAS
I previously discussed the technical nuances for the microscope-guided approach in the chapter on Microscope-Guided Endonasal Transsphenoidal Approach of the Cranial Approaches volume. Moreover, the endoscopic-guided route is discussed in the Endoscopic Expanded Transnasal Approach. Please refer to these chapters for information regarding the initial and final stages of the operation, including exposure and closure.
Although I routinely employ the endoscopic route for resection of pituitary microadenomas, the details for both endoscope and microscope-assisted tumor resection are discussed herein. The benefits of endoscopic surgery and its wider working zone are less appreciable for microadenomas than for macroadenomas because most microadenomas do not extend beyond the sella and are relatively confined to the gland.
Figure 2: When bone removal at the floor of the sella is complete, the exposed dura, including the intercavernous sinus, is cauterized with bipolar electrocautery. I use an ample amount of Floseal hemostatic matrix (Baxter, Deerfield, IL) to seal the bleeding from the cavernous sinus and other venous lakes within the exposed dura. Aggressive coagulation of the dura may lead to its shrinkage and excessive epidural bleeding.
The principle of judicial minimalism in bone removal, while not compromising adequate lesional exposure to conduct microsurgery and tumor removal, is pertinent. The most common cause of subtotal tumor resection is inadequate bone removal and a resultant restricted dural opening. Bony removal should unroof the medial walls of the cavernous sinuses and be bounded by the tuberculum and dorsum sellae. Wide exposure is mandatory as gross total resection of functioning tumors is mandatory for effective cure.
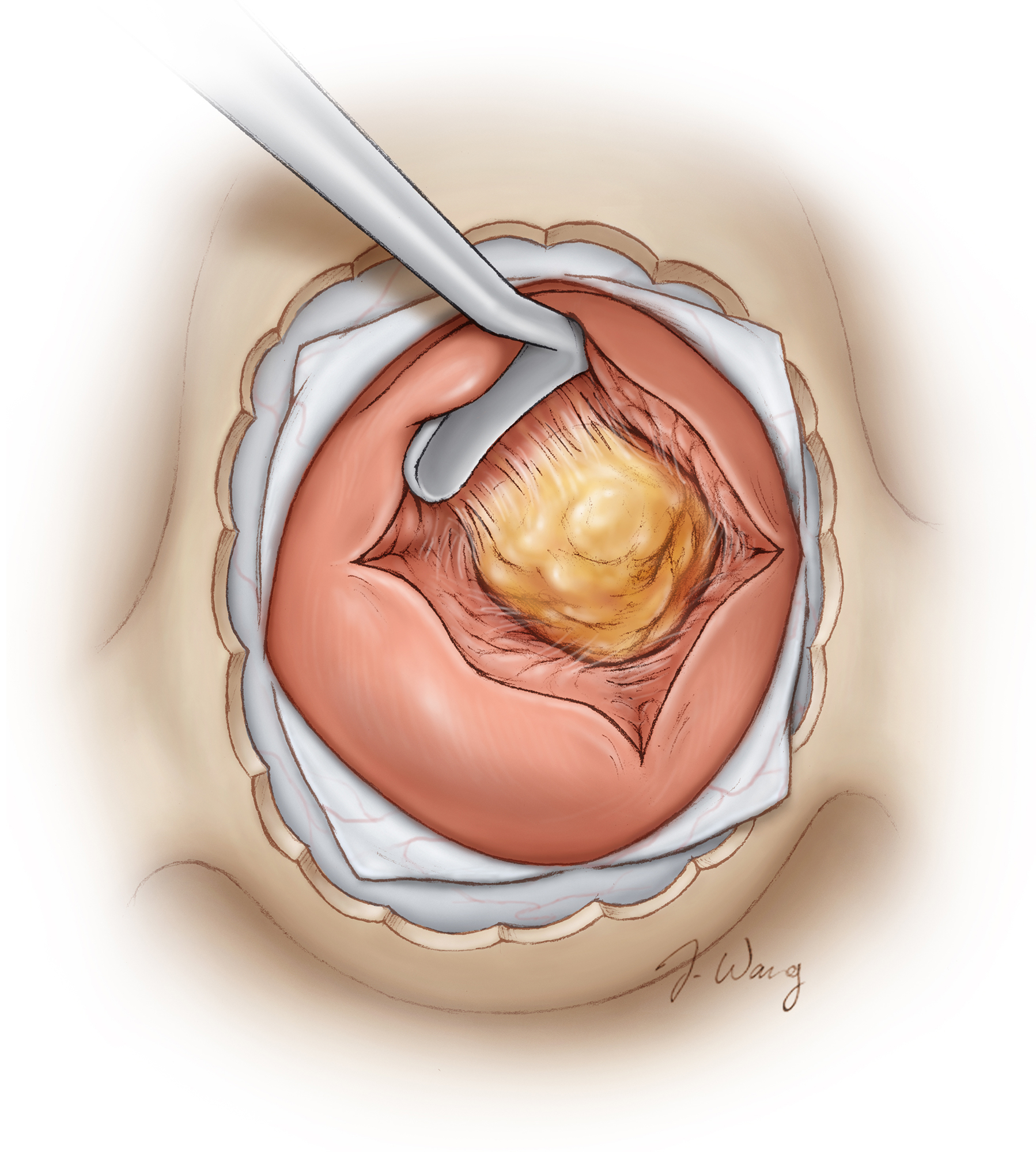
Prior to dural opening, a circumferential bony edge should be mucosa-free to allow reconstruction of the floor at the end of the operation. Ultrasonography may be used to guide the dural opening and avoid injury to the carotid arteries. The dura is incised in a cruciate fashion, and a round dissector is used to widely dissect the pituitary capsule from the inner surface of the dura. This maneuver expedites identification of the capsule during the later stages of the operation (Redrawn from Tew, van Loveren, Keller*).
INTRADURAL PROCEDURE
The following offering describes resection of a microadenoma through both microscope- and endoscope-assisted methods. For a more detailed discussion of the advantages and disadvantages of microscopic versus endoscopic adenoma resection, see Pituitary Adenoma: Diagnosis and Operative Considerations.
Figure 3: The initial cruciate incision within the gland exposes the central microadenoma in Cushing’s disease. Localizing the microadenoma can be quite difficult depending on its size and location within the parenchyma. If the adenoma is not readily seen, a systematic approach is used to inspect the gland’s parenchyma. In acromegaly, the microadenoma is laterally located and is more gelatinous; these features facilitate its identification and removal upon opening of the dura (Redrawn from Tew, van Loveren, Keller*).
The distinction of normal from neoplastic glandular tissue can be quite difficult in some cases. The microadenomatous tissue often appears more yellow than the gland. Moreover, the texture and integrity of the neoplastic tissue is friable and can often be removed by suction or curettage.
However, microadenomas can present without any identifiable surface features to distinguish them from the normal gland. Small tumors (<5 mm) are commonly found in the middle third of the gland. This configuration is encountered with adrenocorticotropic hormone (ACTH)-secreting adenomas, as opposed to prolactin-secreting or growth hormone-secreting adenomas, which are more often located laterally within the gland. When there are no identifiable features suggestive of the location of the microadenoma, this configuration can guide the initial dissection within the gland. Prolactin-secreting or growth hormone-secreting adenomas are almost always easily identifiable and are very soft and suctionable. This is in contrast to ACTH-secreting microadenomas, which are firmer, more infiltrative and embedded within the center of the gland.
If an ACTH-secreting microadenoma is not readily seen upon dural incision, a systematic approach to inspection of the gland as described above is warranted. If this search is nonconclusive, a biopsy of the central mucoid wedge should be considered. This can be followed by biopsy of the lateral wings if no evidence of an adenoma is found on the initial central biopsy. High-resolution 3T magnetic resonance imaging or petrosal venous sampling often guides the surgeon to biopsy the suspicious location or side.
If these biopsies prove unremarkable and the biochemical diagnosis of Cushing’s disease is unquestionable, the surgeon may perform a subtotal hypophysectomy, leaving only a small pedicle of the original adenohypophysis. This approach is applicable only if future fertility is not a concern for the patient. However, if the resected mass does not house the microadenoma, the surgeon may consider extending the resection to include the neurohypophysis and/or bilateral cavernous sinus walls.
The microadenoma has been shown to rarely exist within the suprasellar region; however, in the absence of radiologic evidence, incision of the diaphragm is generally not indicated or recommended.
Although I have performed subtotal hypophysectomies on a few patients with unquestionable biochemical confirmation of Cushing’s disease, I have not attempted more aggressive resection.
Figure 4: After the microadenoma is identified, a micro-enucleator circumferentially separates the microadenoma from the adjacent normal glandular tissue. If the adenoma is not immediately seen when entering the gland, a Hardy dissector may be used to apply lateral pressure within the incision to force the microadenoma into the surgical field. Note the color difference between the microadenoma and the gland. I meticulously attempt to preserve the tumor pseudocapsule (extracapsular dissection) and remove the tumor en bloc (Redrawn from Tew, van Loveren, Keller*).
Removal of a microadenoma within the anterolateral portion of the gland is technically difficult because of the inaccessible location of the anterior sellar recess. A microprolactinoma can hide in this location. I reach this region using angled endoscopes and a right-angled microcurette with a longer shaft. Venous bleeding from disruption of the capsule vessels that drain toward the adjacent cavernous sinus is a frequent complication with this technique. To achieve hemostasis, I use thrombin-soaked Gelfoam to pack the area. One should not attempt to coagulate this bleeding source because this tactic almost always leads to more bleeding.
Posteriorly located microadenomas can also be difficult to reach. Resection of a posteriorly located microprolactinoma begins with wedge resection of the anterior segment of the lateral wing, preserving the normal tissue to allow histologic comparison. The micronodule can occasionally appear grayish-purple or white. I begin the dissection with a blunt ring curette or enucleator and then transition to using a sharp ring curette to improve precision of my dissection. The tumor capsule should be preserved to ensure complete excision of the nodule.
Following dissection of the tumor, the resection cavity should be carefully examined for the presence of normal gland, which appears orange. This inspection verifies complete tumor resection. If there is any concern for subtotal resection or if the tumor capsule is indistinct in certain locations, a graded partial lobectomy can be completed. This lobectomy involves resection of a small portion of the underlying normal pituitary parenchyma along the suspicious aspect of the resection cavity.
As was noted in the approach to resection of posteriorly located adenomas, partial extirpation of the normal gland during uncapping the tumor may be required to completely remove the adenoma. This is generally well tolerated by the patient without lasting endocrine deficits. Avoidance of this maneuver can overlook residual hypersecreting tumor and is associated with long-term undesirable consequences. Substantial (>80%) alteration within the normal pituitary parenchyma leads to impairment in one or more of the endocrine axes.
Figure 5: Following circumferential dissection of the microadenoma, the mass can then be removed. The capsule of the nodule is preserved and the nodule is removed en bloc. The resection cavity is carefully inspected (Redrawn from Tew, van Loveren, Keller*).
Figure 6: The wall of the resection cavity may be removed and pathologically examined. This maneuver improves the odds of complete endocrine remission (Redrawn from Tew, van Loveren, Keller*).
Figure 7: A sharp ring curette can then be inserted within the incision to aid in extraction of the peritumoral sample.
Closure
Minor CSF leakage after removal of microadenomas is not uncommon.
Figure 8: Minor manipulations of the gland can lead to disruption in the attachments of the diaphragm to the anterior sellar recess. This disruption leads to small areas of cerebrospinal fluid leakage. Therefore, the resection cavity is packed with autologous adipose tissue. Using this maneuver, I attempt to elevate/mobilize the gland and buttress/seal the diaphragmatic defect. Overpacking is avoided. A piece of bone or prosthesis is used to reconstruct the sellar floor and keep the fat in place (Redrawn from Tew, van Loveren, Keller*).
Case Example
This patient presented with Cushing's disease and underwent endoscopic transsphenoidal resection of her microadenoma. The following images demonstrate the intraoperative events during gross total removal of the tumor for biochemical remission.
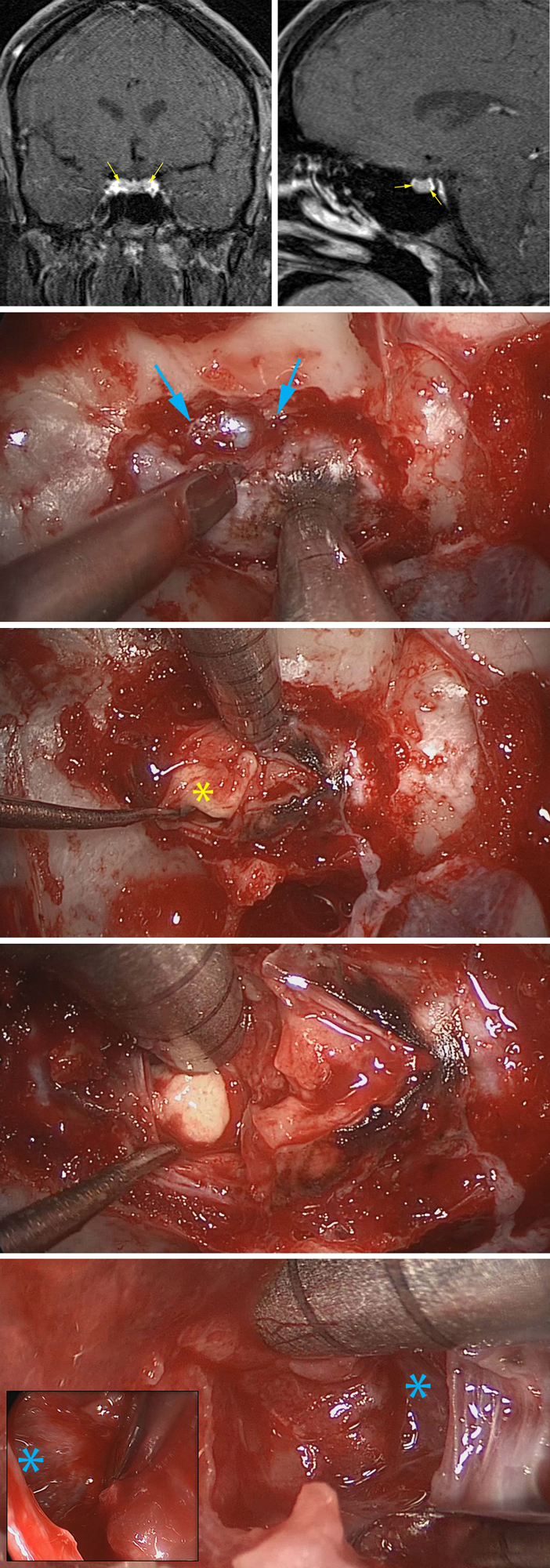
Figure 9: The preoperative MR images define the central location of the adenoma (top row). Note the generous bony removal along the floor of the non-expanded sella; the medial edge of both cavernous sinuses and the intercavernous sinus (blue arrows) are unroofed (second row). An incision in the gland and extracapsular dissection deliver the majority of the tumor (*)(third row). A hidden portion of the tumor just medial to the wall of the cavernous sinus is extracted (fourth row). The wall of both cavernous sinuses are inspected (*) for confirmation of complete adenomectomy (last row).
Microadenomectomy for Cushing's Disease
Microadenomectomy for Cushing's Disease: Maximizing Resection
Postoperative Considerations
For a more detailed description of postoperative considerations, see the Pituitary Adenoma: Diagnosis and Operative Considerations chapter.
The success of resecting an ACTH-secreting microadenoma can be evident on laboratory analysis between postoperative days two and three. Standard laboratory evidence suggestive of a complete resection includes an undetectable serum ACTH level and morning cortisol <5 mcg/dl. Importantly, if the serum cortisol level decreases dramatically postoperatively but remains within the normal physiologic range, it generally indicates an incomplete resection. For these patients, a re-exploratory transsphenoidal operation is reasonable if the diagnosis of Cushing’s disease is biochemically unquestionable. Other options for further therapy include medical and radiation therapy as well as a bilateral adrenalectomy.
The patients suffering from Cushing’s disease should be monitored in the intensive care unit postoperatively so that their acute syndrome of hypocortisolism can be quickly diagnosed and treated. This diagnosis is a desirable finding as it almost always signifies biochemical cure.
Remission from acromegaly has been defined as a postoperative serum insulin-like growth factor-1 (IGF-1) level within normal limits and a growth hormone (GH) <1 ng/ml when drawn during an oral glucose tolerance test (OGTT). Historically, a basal GH <5 ng/ml may indicate a surgical cure; however, this measure has been questioned. By meeting the remission criteria above, the basal GH level is nearly universally <5 ng/ml; however, this is not a reciprocal relationship. Therefore, assessment of remission should include an OGTT GH and IGF-1 measurements.
Pearls and Pitfalls
- ACTH-secreting microadenomas can be difficult to identify and completely resect. The gland should be explored systematically to find an occult small microadenoma. These patients’ lives can be dramatically improved through a curative resection.
Contributor: Benjamin K. Hendricks, MD
For additional illustrations of using endoscopes during skull base surgery, please refer to the Jackler Atlas by clicking on the image below:
*Redrawn with permission from Tew JM, van Loveren HR, Keller JT. Atlas of Operative Microneurosurgery, WB Saunders, 2001. © Mayfield Clinic
Please login to post a comment.