Transcallosal Expanded Transforaminal Transvenous Transchoroidal Route
Walter Dandy established the safety of surgery in the third ventricle. He also introduced cerebral pneumoventriculography, which paved the way for reliable diagnosis of ventricular tumors. With his daring personality, he pushed the conservative boundaries of neurosurgery. One of Dandy’s contributions was the technique of transecting normal frontal lobe tissue to uncover deep-seated lesions
Figure 1: Walter Dandy described partial resection of the frontal lobe to reach an anterior third ventricular tumor. He described the tumor as “cystic containing calcified concretions” (Dandy WE. The Brain. Hagerstown, MD: Prior Company, 1966).
This is a preview. Check to see if you have access to the full video. Check access
Resection of a Large Third Ventricular Tumor (Chordoid Glioma) via the Transcallosal Expanded Transforaminal Transvenous Route
The specific region of focus in this discussion is the anterior segment of the third ventricle. The region between the optic chiasm and the foramen of Monro approximates this portion of the chamber. The boundaries of this region include the optic chiasm, lamina terminalis, anterior commissure, and columns of the fornix.
Creation of safe operative corridors and microsurgical handling of the underlying lesion to avoid any injury to the walls of the vital surrounding structures are the founding principles that make ventricular surgery rewarding. Appropriate patient selection is also crucial to desirable outcomes.
Surgical Approaches to the Anterior Third Ventricle
There are three different approaches to the anterior third ventricle that are appropriate for surgical access to this region. In my opinion, the interforniceal route places the fornices and memory at risk, so the following first two approaches are most reasonable:
- Transcallosal expanded transforaminal transvenous transchoroidal route
- Subfrontal or endoscopic endonasal translamina terminalis route
- Transcallosal interforniceal route
Other operative routes to the third chamber are discussed in the Introduction to Third Ventricular Tumors chapter.
Diagnosis and Evaluation
For a general discussion of diagnosis and evaluation for ventricular tumors, see the Principles of Intraventricular Surgery chapter.
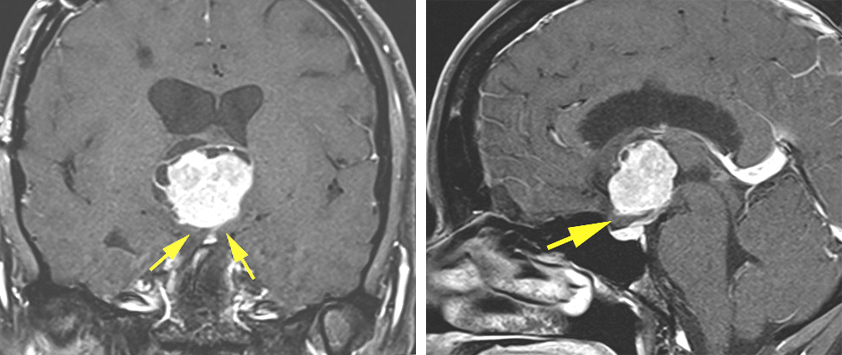
Figure 2: An anterior third ventricular chordoid glioma is pictured. This tumor had been previously approached via a transfrontal route at another institution. Because of the limited working angles of this approach, resection was abandoned at that time. I approached this tumor via a transcallosal expanded transforaminal transvenous route after disconnecting the septal vein and dissecting the anterior choroidal fissure. Note the location of the optic chiasm underneath the tumor (yellow arrows) and the limited space across the lamina terminalis. These features made the transnasal and subfrontal routes prohibitive.
Indications for Surgery
For a general discussion of the indications for surgery, see the Principles of Intraventricular Surgery chapter.
The transcallosal expanded transforaminal transvenous trajectory is my preferred approach to reach anterior and mid third ventricular tumors. If the tumor cannot be reached through the endoscopic transnasal translamina terminalis corridor, I use the transcallosal expanded transforaminal transvenous route. These two approaches can handle more than 95% of the third ventricular tumors. Most third ventricular craniopharyngiomas have at least a suprasellar connection and are suitable for an endonasal trajectory.
Large firm, solid and potentially vascular tumors without any associated expansion of the lamina terminalis are more effectively exposed via the transcallosal expanded transforaminal transvenous pathway.
Preoperative Considerations
For a specific discussion of the preoperative considerations for third ventricular tumors, see the Principles of Intraventricular Surgery chapter.
A study of the parasagittal convexity veins using magnetic resonance imaging is useful. I also pay special attention to the anatomy of the ventricular veins (including the thalamostriate veins) and choroid plexus. The planned operative intradural trajectory is carefully studied, and the size of the foramen and any widening of the anterior choroidal fissure determines the side of the approach for a purely midline lesion.
I consider the contralateral cross-court trajectory (working through the right foramen to see the lateral capsule of a left-sided tumor) in select cases. Peritumoral edema is an ominous sign and indicates pial invasion and a need for subtotal resection.
Preoperative hydrocephalus may indicate a need for a ventriculostomy before interhemispheric dissection is contemplated so that this portion of the dissection is conducted smoothly without undue retraction and pial injury.
Figure 3: The pathoanatomy of an anterior third ventricular tumor is illustrated. The size of the ventricle as well as the degree of the expansion of the foramen and anterior choroidal fissure by the tumor determine the side of the interhemispheric operative trajectory.
Figure 4: Suprasellar connection of the tumor and expansion of the lamina terminalis determine the suitability of the transnasal versus transcallosal route. Recurrent tumors are best approached via an intact operative trajectory.
Operative Anatomy
For relevant operative anatomy, please refer to the chapter on the Anatomy of the Ventricular System.
TRANSCALLOSAL EXPANDED TRANSFORAMINAL TRANSVENOUS TRANSCHOROIDAL ROUTE
The lateral head position with the sagittal suture parallel to the floor is most reasonable for using gravity retraction. Although I use this position for exploration of the lateral ventricle, I use the neutral or “nose up” head position for third ventricular tumors. The falx is not present in the third ventricle to prevent the contralateral ventricular contents from collapsing into the operative field.
Figure 5: The patient’s body may be placed in the supine (left image) or lateral (middle image) position to place the head in the lateral configuration. Many colleagues prefer the supine position (right image) so that the midline is anatomically situated, avoiding surgical disorientation at the deeper levels of the dissection.
Figure 6: The borders of the skin incision and craniotomy are outlined. The craniotomy is often centered over the coronal suture. I prefer a linear incision over a horseshoe incision and a relatively small craniotomy. This exposure is more than adequate to conduct microsurgery at the deep corridors.
The bone flap is typically made on the right side, and is approximately 5-6 cm in the anterior-to-posterior direction, either centered on the coronal suture or two-thirds anterior (4 cm) and one-third (2 cm) posterior to the suture, depending on whether a more posteriorly directed trajectory is required.
Single burr holes may be placed on the superior sagittal sinus (using navigation) or a pair on either side. The dura is opened in a curvilinear fashion with the base located along the superior sagittal sinus. It may be necessary to sacrifice one, maybe two, small bridging veins to enter into the interhemispheric fissure. This maneuver is usually safe in this region. Larger veins should be untethered but preserved.
The details of the craniotomy and interhemispheric dissection are described in the Interhemispheric Craniotomy and Lateral Ventricular Tumors chapter.
Figure 7: The operative anatomy of the choroid plexus, septal and thalamostriate veins are depicted in these illustrations. Transection of the septal vein at the point where it joins the thalamostriate vein is the key step in expanding the foramen via a small anterior transchoroidal dissection. The fornices and thalamostriate veins are strictly preserved. Although I prefer to dissect the choroidal fissure along its thalamic side rather than its forniceal side to protect the fornix, the anatomy of the fissure is more likely to dictate the optimal side of dissection. In other words, I do not recommend a uniform dissection strategy (always along the taenia thalami versus the taenia fornix). The fornix forms the borders of the anterior wall of the foramen and should be protected during movements of the instruments.
Figure 8: The various operative viewing angles (green area) versus blind spots (red area) for the transforaminal transvenous transchoroidal approach is illustrated.
INTRADURAL PROCEDURE
In summary, the transcallosal approach exposes the ipsilateral lateral ventricle. Next, the foramen of Monro is enlarged through transection of the septal vein and its disconnection from the thalamostriate vein. This maneuver allows enlargement of the foramen through a minimal anterior transchoroidal dissection without significant manipulation of the thalamus or thalamostriate vein. Asymmetrical tumors can be tackled through a contralateral transforaminal entry (cross-court route) to reach the lateral aspect of the tumor.
Figure 9: Neuronavigation is critical to orient the surgeon with respect to the desired trajectory to reach the foramen through a corpus callosotomy of appropriate location and minimal length (left image). A 2cm callosotomy is situated just to the right of the midline, exposing the right lateral ventricle (right image). Release of cerebrospinal fluid at this juncture further relaxes the frontal lobe. Note the sutures in the superior falx that mobilize the superior sagittal sinus.
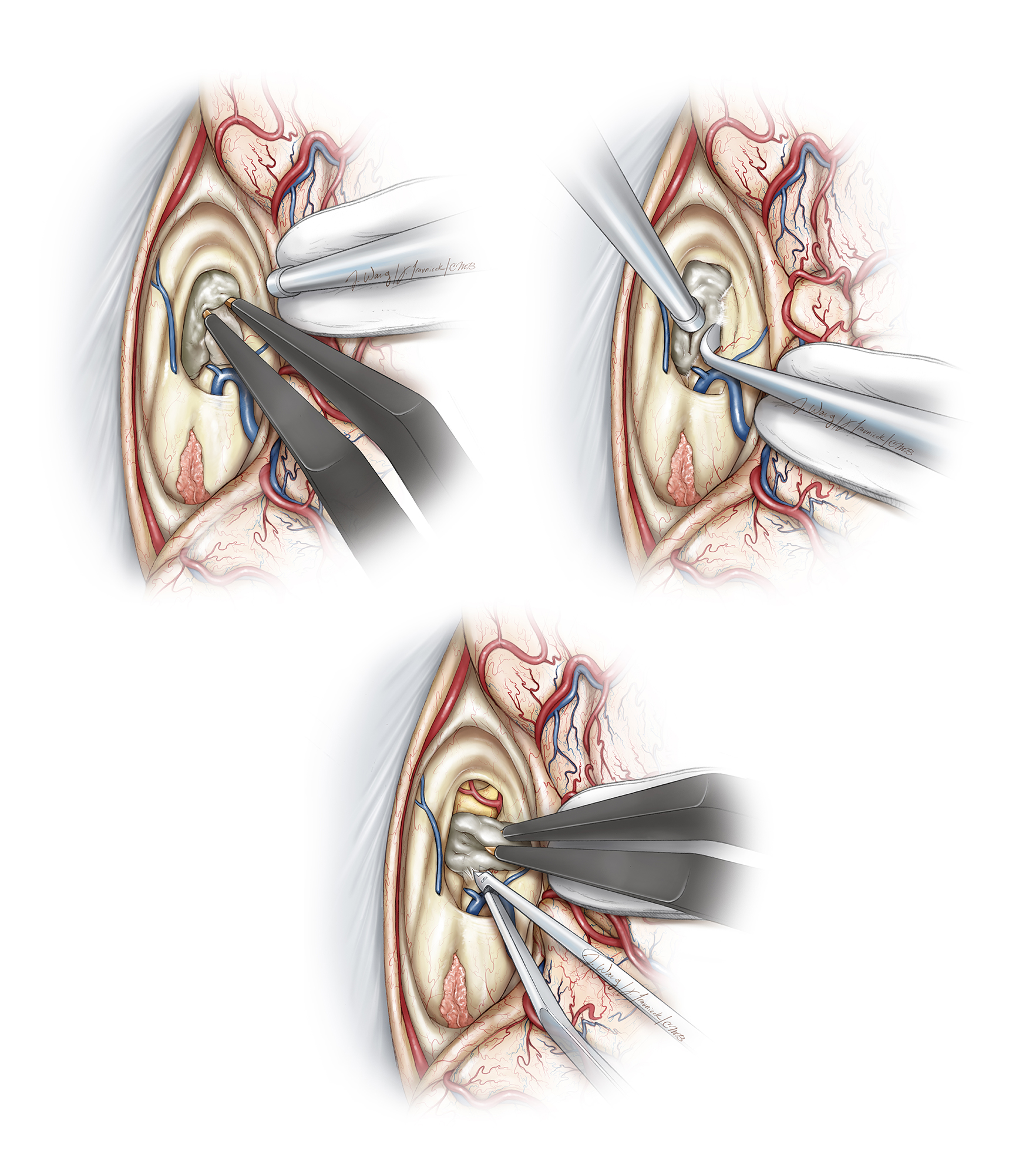
Figure 10: The anterior section of the choroid plexus overlying the foramen of Monro and the venous angle is gently and bluntly mobilized and coagulated. The tumor often expands the foramen, but does not provide an adequate operative corridor to allow gross total removal of the mass (Redrawn from Tew, van Loveren, Keller*).
Figure 11: The proximal section of the septal vein at the foramen where it joins the thalamostriate vein is skeletonized, coagulated, and cut (upper illustration). An intraoperative photo of the right ventricle and the relevant operative anatomy is provided (lower image).
Figure 12: The transection of the anterior septal vein and transchoroidal dissection posterior to the thalamostriate vein provides excellent exposure of the anterior and middle portions of the third ventricle and the tumor. After the anterior septal vein is coagulated and sharply cut, the fornix is gently mobilized medially. There is usually a small section of ependyma between the posterior edge of the foramen and the anterior margin of the ipsilateral thalamostriate vein. This ependyma can be dissected open with micro dissectors and by gentle spreading the bipolar forceps (upper image). Collectively, these maneuvers significantly expand the foramen, facilitating improved visualization through the third ventricle (lower intraoperative photo). The left third ventricular wall is marked with *.
The dissection along the medial wall of the ipsilateral internal cerebral vein may be extended posteriorly to further expand the operative corridor into the third ventricle. Bilateral internal cerebral veins will eventually emerge, and the posterior capsule of the lesion is readily exposed through this route.
Figure 13: Before I debulk the tumor, I orient myself with respect to the walls of the ventricle so that I do not inadvertently penetrate the capsule during debulking maneuvers. I mobilize the tumor capsule from the superior walls of the ventricle (left upper image). Next, the tumor is enucleated using ultrasonic aspiration and its capsule is dissected away from the lateral walls (right upper image). These steps are repeated until the tumor capsule is sharply dissected off of the floor (lower image). If the tumor has eroded the floor of the third ventricle, the optic nerve and chiasm will often be apparent at the bottom of the resection cavity (Redrawn from Tew, van Loveren, Keller*).
Figure 14: This illustration demonstrates the intact volume of the foramen of Monro and the anatomy of the ventricle through the transcallosal route. The following two illustrations demonstrate the increase in the operative corridor through the expanded foramen upon transection of the septal vein and minimal anterior choroidal dissection.
Figure 15: The first step of the transection of the septal vein leads to an immediate expansion of the foramen. Even without any choroidal dissection, coagulation and resection of the very anterior choroid plexus leads to a generous inlet into the third ventricle. In this view, the microscope is directed along the anterior floor of the third ventricle.
Figure 16: Tailored anterior choroidal dissection and strategic direction of light into the posterior ventricle allows a complete panoramic viewing within the third chamber. These steps are designed to avoid unnecessary choroidal dissection and resultant potential forniceal injury (otherwise needed if the exposure through the foramen is not exploited and the traditional transchoroidal route is utilized without septal vein sacrifice.)
In summary, the tumor is first internally debulked and then anteriorly, laterally, posteriorly, and finally inferiorly dissected along its capsule.
The velum interpositum does not need to be entered unless necessary. Once the mass is removed, the aqueduct can be visualized and an angled mirror may be placed into the third ventricle to inspect the contralateral foramen of Monro. I frequently fenestrate the septum pellucidum.
Other Considerations
The most difficult part of dissection may be underneath the contralateral fornix because the ipsilateral fornix can hide this section of the operative corridor. It is imperative to avoid any pial transgression of the walls or the floor. Adherent sheet of tumor along the floor should be left behind to protect the hypothalamus unless the tumor has already destroyed the integrity of the floor. Aggressive manipulation of the internal cerebral veins is hazardous and fraught with undesirable consequences because of their thrombosis. Fixed retractors are not used in the ventricle because they lead to ischemic injury.
Excessive bleeding within the ventricular chambers is avoided and a small piece of cottonoid patty is used to plug the lateral ventricular pathways around the resection cavity to minimize drainage of blood into the distal cavities. Importantly, pristine dissection planes will minimize injury to the ventricular walls. The suction device should not be directly placed on these walls. The tumor should be mobilized away from the wall rather than vice versa.
The forniceal bodies affect short-term memory and are very sensitive to any manipulation. Both fornices are equally important; neither of them can be sacrificed. This principle also applies to the thalamostriate and internal cerebral veins.
The tumor should be removed piecemeal using an ultrasonic aspirator. The surgeon should not attempt to handle or pull on large tumor fragments because this maneuver often exerts excessive traction forces on the walls. Strict adherence to gentle microsurgical techniques is the foundation for operative success within the chamber that is considered the “seat of the soul.”
Closure
For moderately to severely vascular tumors or when intraventricular bleeding has been encountered intraoperatively, I place an external ventricular catheter into the resection cavity before closure.
All ventricular cavities are generously irrigated and inspected to remove blood clots. Finally, the ventricle and subdural spaces are filled with fluid to eliminate as much air as possible.
Postoperative Considerations
For general principles of postoperative care after ventricular surgery, please refer to the chapter on the Principles of Intraventricular Surgery.
Pearls and Pitfalls
- Gentle manipulation of neurovascular structures is imperative during surgery of the third ventricle. Surgical experience in this area leads to a conservative operative behavior.
Contributor: Benjamin K. Hendricks, MD
*Redrawn with permission from Tew JM, van Loveren HR, Keller JT. Atlas of Operative Microneurosurgery, WB Saunders, 2001. © Mayfield Clinic
Please login to post a comment.