Microscope-Guided Endonasal Transsphenoidal Approach Free
General Considerations
The endonasal transsphenoidal approach has evolved during the past century. Harvey Cushing played an instrumental role in popularizing the endonasal route as it was pioneered by surgeons of his time, including Schloffer and Halstead. During the infancy of cranial surgery, transsphenoidal surgery literally placed Cushing on the brink of exploring brain surgery.
Figure 1: Cushing maintained an intense interest in pituitary surgery throughout his career. In these rare photos of Cushing with a patient, he demonstrates the features of acromegaly (Courtesy of the Cushing Brain Tumor Registry at Yale University).
Figure 3: By 1929, Cushing had excelled in transcranial techniques and abandoned the transnasal route in favor of the subfrontal approach. In his notes, he documented the incision (left) and operative findings (right) for the subfrontal approach to a craniopharyngioma (Courtesy of the Cushing Brain Tumor Registry at Yale University).
As lighting, endoscopy, and instrumentation evolved during the past decades, so have the surgical limits of what is possible through the endonasal corridor. The transsphenoidal approach has become the preferred route of access to parasellar lesions such as pituitary adenomas, meningiomas, and craniopharyngiomas. Endoscopy has obviated the need to resect pituitary tumors through the transcranial corridor. In contrast to the pterional and orbitozygomatic approaches, the transnasal approach eliminates the need for brain retraction.
In the past few years, the endonasal route has been used in conjunction with endoscopy to access the anterior cranial base, parasellar, and paraclival regions. In this chapter, I review the tenets for the microscopic transnasal approach. Since our ear, nose, and throat colleagues often perform the endoscopic transnasal exposure for us, I will not address the corresponding nuances for the endoscopic approach in this Atlas. However, neurosurgeons continue to perform the transnasal exposure using an operating room microscope, and this chapter is dedicated to the description of this technique. The neurosurgical methodologies for resection of skull base tumors through the endoscopic transnasal route are discussed in the Pituitary and Parasellar Tumors chapters of the Brain Tumors Volume.
Indications for the Approach
The microscopic endonasal transsphenoidal approach is ideal for reaching lesions confined to the paramedian sellar and suprasellar territories medial to the carotid arteries and inferior to the subchiasmatic space. Substantial vertical growth of the tumor is not a contraindication as long as the tumor does not have extensive lateral dispersions beyond the limits of the cavernous sinus. Common lesions treated via microscopic transnasal surgery include pituitary adenomas, Rathke’s cleft cysts, and selected parasellar meningiomas, craniopharyngiomas, and clival chordomas. The addition of endoscopy radically expands the reach of the transnasal exposure to access lesions beyond the sella and along almost the entire skull base.
By utilizing an endoscope, the surgeon can gain an expanded view that reaches the anterior skull base for orbital groove, planum sphenoidale, and tuberculum sella meningiomas. Third ventricular craniopharyngiomas, paraclival chordomas, and condrosarcomas are easily uncovered using endoscopic endonasal surgery. For tumors with significant suprasellar and middle fossa extension, I attempt an initial resection through the transnasal route and prepare the patient for a second stage transcranial surgery.
Preoperative Considerations
Any parasellar lesion demands that the patient receives a through pituitary axis hormonal evaluation to rule out perioperative inadequate pituitary reserve. The patient’s serum prolactin, cortisol, and thyroxine levels are especially important. Routine use of perioperative glucocorticoids has eliminated intraoperative hypocortisolemia, but pre-existing hypothyroidism may present acutely after surgery. I have been surprised by very unusual asymmetric imaging presentations of prolactinomas centered somewhat away from the midline.
Figure 4: Atypical presentation of a prolactinoma in a patient with seizures. Please note the laterality of the mass and its resemblance to a meningioma on imaging. This case underlines the importance of preoperative evaluation of the prolactin level to avoid unnecessary surgery.
It is critical that the surgeon has a clear understanding of the location of the carotid arteries. The ectatic arteries may be misplaced to the midline and easily injured during the dural opening and later dissection. Invasive tumors can destroy bony landmarks along the sella and significantly disorient the operator.
Figure 5: Note the location of the right cavernous carotid artery displaced by this invasive pituitary adenoma (top images). The tumor had eroded through the skull base and no reliable landmark was found intraoperatively. I inadvertently lacerated the carotid artery during the dural opening. The initial torrential bleeding was controlled with gentle pressure over the visible laceration site using a piece of cotton. Tumor resection was continued since this patient was suffering with significant progressive visual decline (immediate postoperative images: bottom images).
If the exact site of an arterial injury cannot be immediately discovered and sealed, the operative field should be packed with cotton to control bleeding, surgery aborted, and an arteriogram performed immediately to rule out a pseudoaneurysm.
Neuronavigation (CT guided) or at least lateral fluoroscopy is especially instrumental in successful execution of the microscopic transnasal route. Invasive tumors and scarring from previous operations can distort normal landmarks and easily disorient the operator. An inappropriate trajectory can lead to misguided bone removal at the skull base and place neighboring cerebrovascular structures at great risk.
A prophylactic lumbar drain may be placed intraoperatively if a repeat operation or procedure with a high risk of postoperative CSF leakage is planned. The patient’s abdomen is routinely prepared for fat graft harvest.
I plan a “cross-court” operative working angle. In other words, tumors with a left-sided suprasellar extension are approached from the right nostril. This principle expands the working angle toward the contralateral hidden suprasellar corners.
Operative Anatomy
Figure 6: Notice the bony prominences created by the carotid artery and optic nerves and the opticocarotid recess. Neuronavigation can be very helpful in delineating the position of these vital structures in the operative field, especially if bony landmarks are not well defined or invasive tumors have altered these landmarks. Note that medially positioned ectatic carotid arteries are contraindications for this approach (Image courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
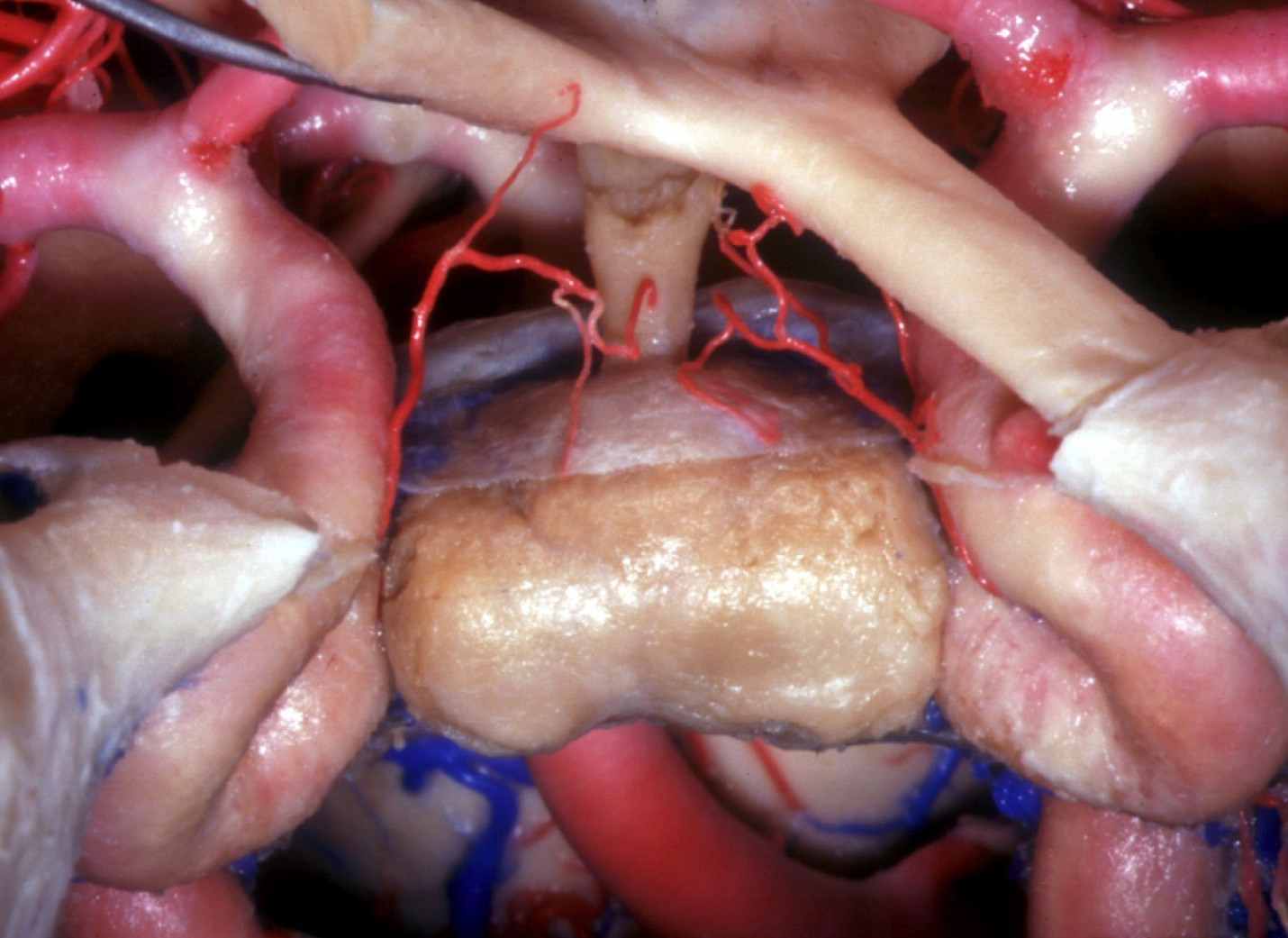
Figure 7: The position of the pituitary gland itself may vary within the sella. The photo shows an anatomical view via the endonasal transsphenoidal approach with the floor and dura of the sella and the cavernous sinus removed. The right optic nerve has been elevated (Image courtesy of AL Rhoton, Jr).
MICROSCOPIC TRANSNASAL CRANIOTOMY
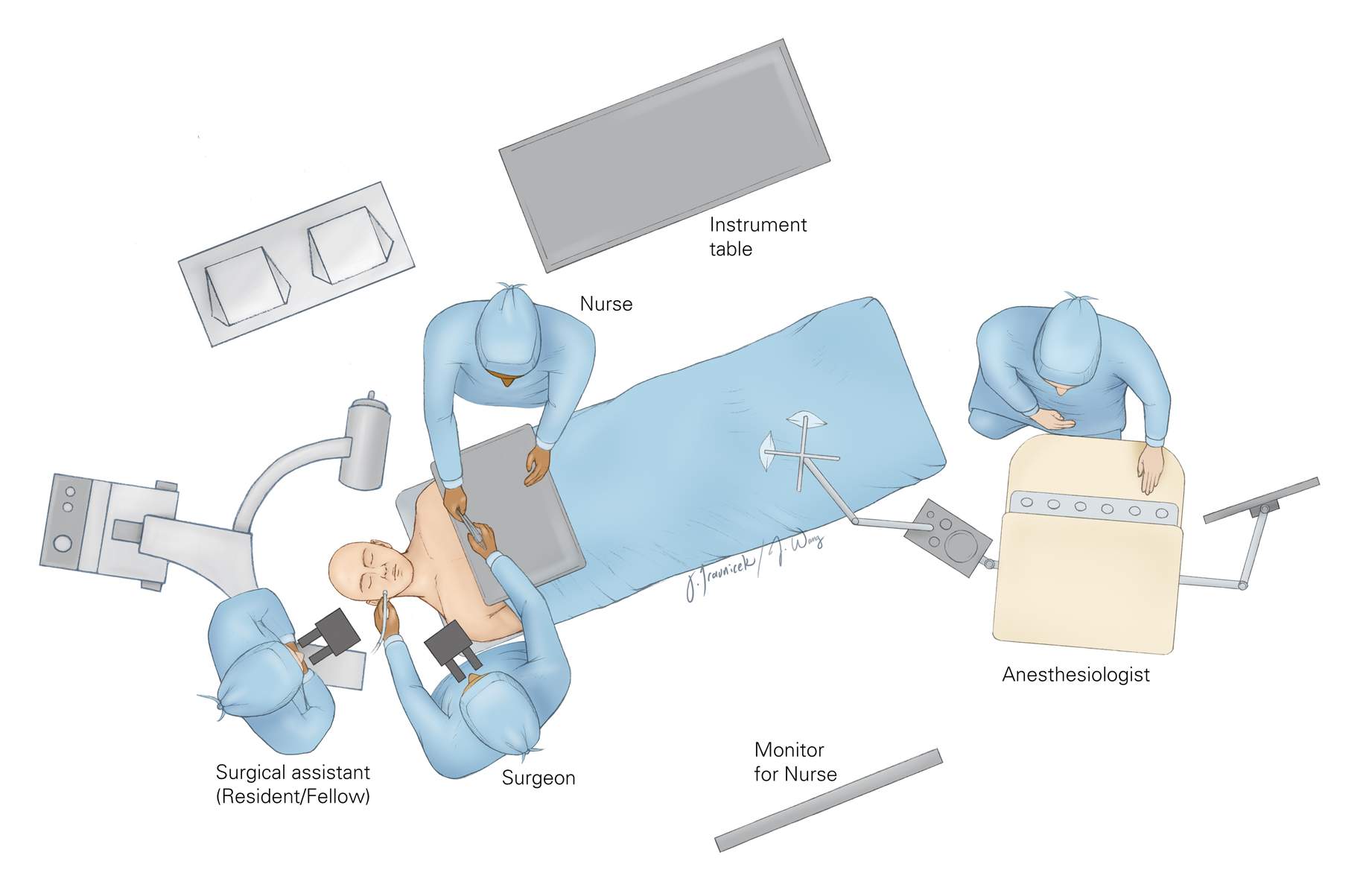
Figure 8: The setup of the operating room depends on the handedness of the surgeon because the surgical technician and the operator are situated across from each other. A right-handed operator is able to readily interact with the surgical technician for the transfer of instruments. The neuronavigation system or fluoroscope is also at the head of the table, across from the surgeon. The anesthesiologist is placed at the foot of the table.
Figure 9: The patient is placed supine in a “sniffing” position with the neck slightly flexed on a horseshoe headrest. Neuronavigation demands the use of a skull clamp. The head fixation limits any later minor intraoperative adjustments in head position.
Figure 10: A right-handed surgeon operates from the right side of the patient. The head is tilted away from the surgeon so that the operator has a straight view of the sellar floor without the patient’s shoulder obstructing the body of the surgeon.
Figure 11: For midline tumors, the nostril with a contralaterally deviated septum and larger size is selected. The operation begins with gentle hydrodissection of the septal mucosa using local anesthesia (xylocaine containing epinephrine) near the anterior quadrangular cartilage or the tip of the vomer bone. This maneuver is hemostatic, and also less traumatic than directly scraping the mucosa off of the septum.
Figure 12: A curvilinear incision is carried in the anterior mucosa. A subperiosteal mucosal incision is developed with a dissector to allow for abundant tissue for a pedicled nasoseptal flap during closure.
Figure 13: A #1 Penfield dissector is used to carry the submucosal incision beyond the septal cartilage and to the level of the vomer. The mucosa should be left intact and even small perforations along the junction of the cartilaginous and bony septi avoided. This flap must be elevated as large as possible while maintaining the integrity of the nasoseptal artery. The quadrangular cartilage (blue) is mobilized to the contralateral side, allowing access to both sides of the vomer and the perpendicular plate of the ethmoid.
Figure 14: A less magnified view of the fracture of the bony or cartilaginous septi and their mobilization laterally (left). The perpendicular plate and vomer can be visualized and must be kept in the midline for purposes of orientation. The contralateral septal mucosa is dissected away from the perpendicular plate of the ethmoid (right). The mucosa must be elevated adequately to expose the sphenoidal crest bilaterally.
Figure 15: The remainder of the midline bony structures are removed with pituitary rongeurs. A bivalve nasal speculum is docked so that the sphenoid crest is midline and the mucosa is retracted laterally (left image). A sagittal view of removal of the perpendicular plate of the ethmoid bone shows the relationship of bony landmarks toward the sphenoid sinus (right image). The dissection is continued superiorly to release the mucosa from the quadrangular cartilage attached to the vomer inferiorly and the perpendicular plate of the ethmoid posteriorly. These maneuvers expose the rostrum of the sphenoid sinus.
Figure 16: A chisel can be used to remove the anterior face of the sphenoid sinus wall to gain access to the sinus mucosa and floor of the sella turcica (left image). Alternatively, Kerrison rongeurs may be used to remove bone starting from both sphenoid ostia. The exact location of the midline and the limits of exposure are confirmed with neuronavigation. The midline septi dividing the sinus into two or more cavities are identified and removed using pituitary rongeurs (right image). An air drill may be used for an underpneumatized sinus. All mucosae within the sinus are exenterated thoroughly.
It is important to emphasize that entry through one nostril deviates the speculum toward the contralateral half of the sella and directly over the corresponding carotid artery. The operator should recognize and resist this natural cross-court deflection and constantly direct the speculum toward the midline.
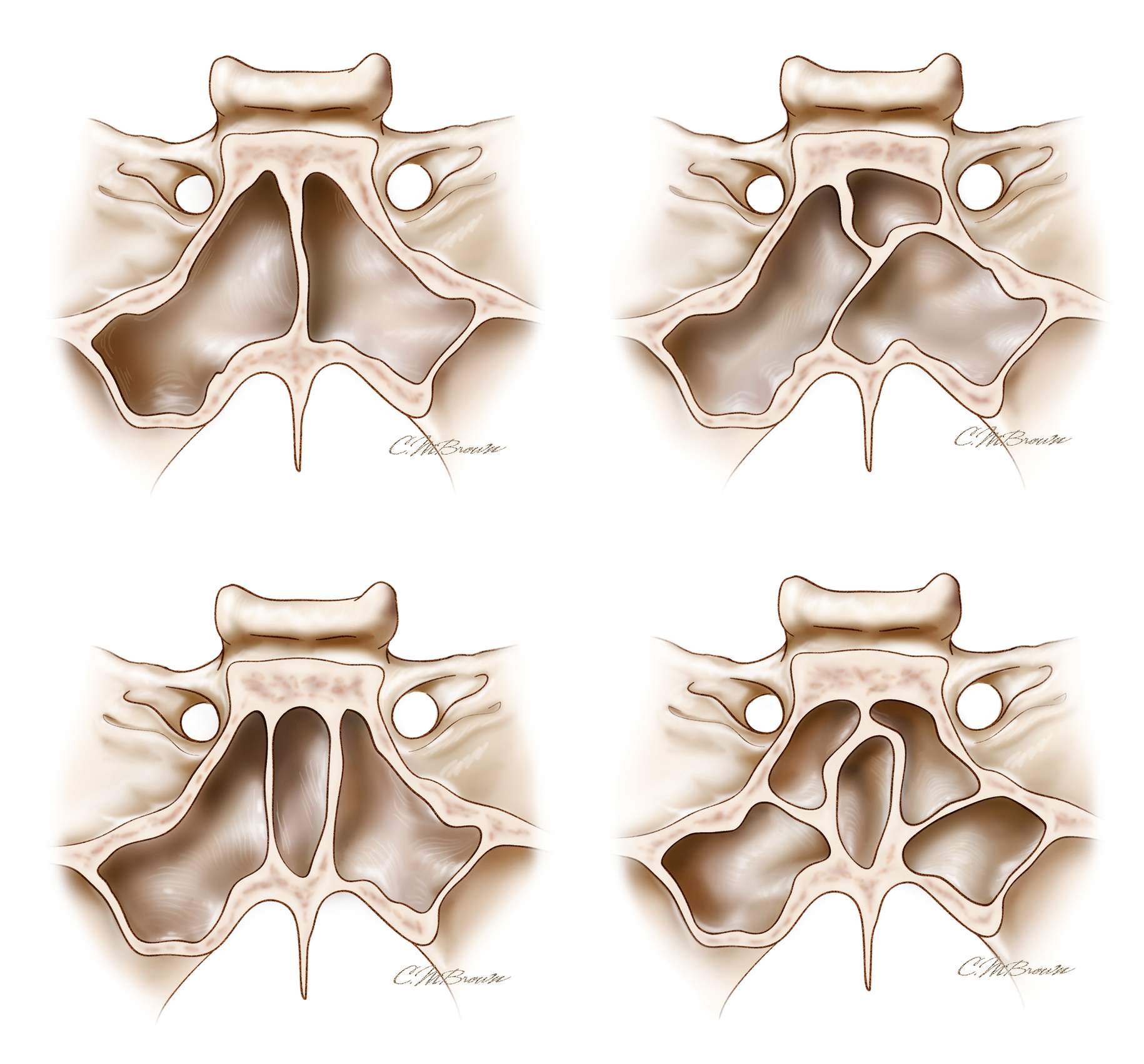
Figure 17: One of the most common mistakes of novice surgeons is a misunderstanding related to the anatomy of the septi in relation to the midline sella. The sinuses with multiple oblique septi seem to cause the most confusion. Some oblique septi connect to the lateral corner of the sella where the carotid artery is protected by only a thin shell of bone.
The most deceiving scenario is when there are two vertical septi located relatively symmetrically from either side of the midline. Removal of one septi gives the false impression that the “midline” septum is removed and the operator is looking at the midsection of the sella. This illusion may lead the surgeon to open the bone over the carotid artery. The usual deflection of the speculum to the contralateral side often complicates this problem further. This erroneous localization of the floor leads to inadequate bone removal and resultant suboptimal tumor resection. Therefore, a clear study of the septal anatomy is necessary before surgery.
Figure 18: The view of the sellar floor through the sphenoid sinus with the carotid prominences visible on the limits of bone removal (left image). A small chisel may be used to remove more bone along the midline sella and allow the use of Kerrison rongeurs to expand the bony opening (right image). Alternatively, if the floor of the sella is thin due to erosion by the tumor, a blunt hook may be used to penetrate and elevate the bone.
I review the patient’s preoperative images to access the location of the carotid arteries because invasive tumors may displace the carotid arteries medially and significantly distort their anatomy.
Figure 19: Further bone removal is cardinal for maximizing tumor removal. In fact, I believe the most important reason for subtotal tumor resection through the transsphenoidal route is suboptimal osteotomy along the floor of the sella, especially laterally. Bone removal should extend to the level of the carotid arteries bilaterally and the tuberculum sella anteriorly.
The dotted red line in this figure represents the cruciate dural opening that gives access for resecting intrasellar and suprasellar lesions. An inadequate dural opening may complicate tumor excision and prevent maximal tumor removal laterally by expanding the operator’s “blind spots.” A hand-held microdoppler probe should be used to guide the dural opening, and if any arteries are displaced medially, the dural opening must be accommodating, otherwise an arterial laceration will occur.
Small venous channels within the double layer of the dura should be sealed with thrombin-soaked gelfoam packing. Aggressive bipolar coagulation leads to an extension of the lateral dural incisions into the cavernous sinus. The tumor is then removed using microscopic techniques. Please see the chapters on non-endoscopic adenoma resection for details about tumor removal.
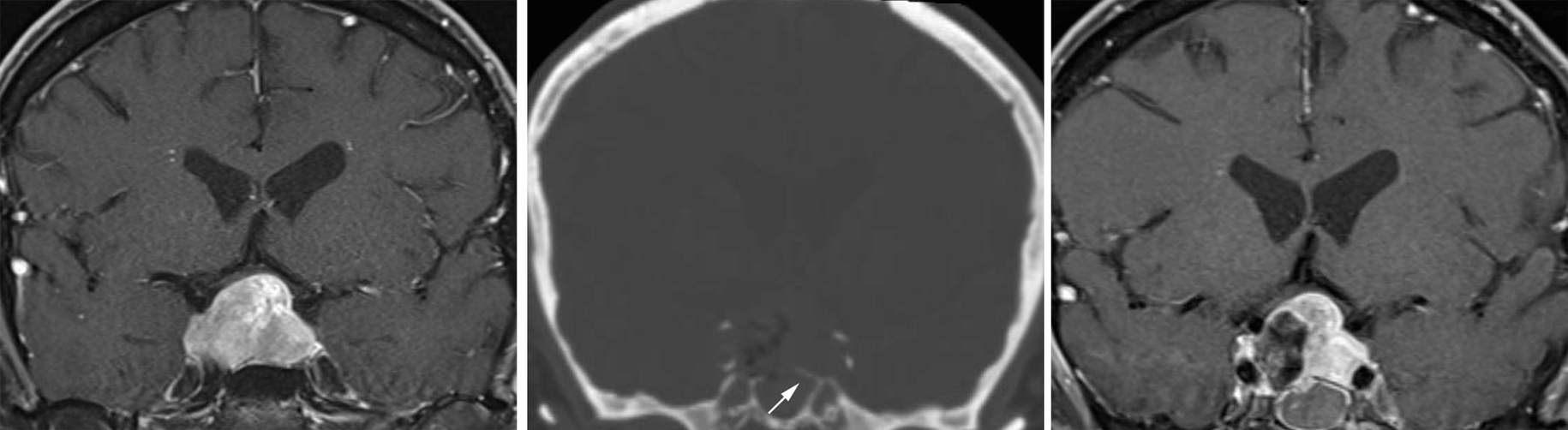
Figure 20: A recurrent pituitary tumor with symptomatic chiasmal compression (left image). Postoperative imaging revealed that subtotal bone removal along the floor of the sella (middle image, arrow) led to inadequate removal of the tumor (right image). The patient was returned to the operating room for additional bone removal and adequate tumor resection.
Closure
Appropriate closure is vital in preventing a postoperative cerebrospinal fluid (CSF) fistula. A fat graft must first be procured from the periumbilical region. I perform this step of the operation at the very beginning of the surgery to avoid subcutaneous contamination of the abdomen by the nasal flora.
I wrap the fat globules with Surgicel. This maneuver allows easy handling of the fat droplets when moving them within the sella and prevents fat from being sucked into the suction apparatus.
The fat is gently packed within the sella to avoid compression of the suprasellar contents. I pack the fat more anteriorly along and above the tuberculum sella because this is the location where occult CSF leaks often occur due to attenuation of the diaphragm sella at the point of its attachment to the anterior sella. The choice of an appropriate method of packing is more important than the amount of packing. Obviously, if there is a visible site of CSF egress within the diaphragma, fat should be packed in the region.
A square segment of a dural replacement material is tucked under the borders of the bony and dural opening along the floor of the sphenoid sinus. If the tear in the diaphragma is large enough to disclose the suprasellar contents, a pedicled septal mucosal flap is rotated onto the defect and a formal skull base reconstruction is completed. A layer of fibrin glue is sprayed over this construct to bolster a watertight seal. The reminder of the bony septum is replaced, the midline mucosae are returned to midline, and both nostrils are packed. I use antibiotic-covered “trumpet drains” to keep the anterior septal mucosae along the midline septum. These drains are removed during the first postoperative day.
Postoperative Considerations
A lumbar drain is implanted and allowed to drain for several days if large defects within the diaphragma were noted intraoperatively. I only selectively monitor patients in the intensive care unit; most patients are monitored on the ward. Patients suffering from Cushing’s disease are observed in the intensive care unit to monitor symptoms of hypocortisolism, which can be life threatening if not addressed quickly. Patients with diabetes insipidus may be managed on the ward if they are awake and cooperative. I consider urine output of more than 400cc for two consecutive hours a possible indication for diabetes insipidus, requiring further investigation with laboratory tests, including urine-specific gravity.
Antibiotics are administered to the patient for as long as the nasal packing is present. A routine postoperative magnetic resonance image is performed to assess the extent of resection. This modality is important to the lifetime training process of the surgeon to improve his or her learning curve in achieving desirable resection outcomes. This learning curve is steep and requires years of experience.
Pearls and Pitfalls
- Neuronavigation (CT guided) is especially important for operations on patients with a poorly penumatized sphenoid sinus, repeat operations, or invasive tumors.
- Misplacement of the nasal speculum into the sphenoid sinus and forceful overzealous spread of its valves leads to serious complications such as anterior skull base fractures and optic nerve injury.
- The extent of bony and dural exposure along the floor of the sella often determines the extent of tumor resection.
- Patience and meticulous reconstruction of the sellar floor avoids disappointing postoperative CSF fistulas.
References
Rhoton AL Jr. The sellar region. Neurosurgery. 2002;51(suppl 1):335-374.
Please login to post a comment.