Chordoma and Chondrosarcoma
This is a preview. Check to see if you have access to the full video. Check access
Petroclival Chondrosarcoma: Endoscopic Endonasal Transclival Transpetrosal Approach
Please note the relevant information for patients suffering from chordoma and chondrosarcoma spasm is presented in other chapters. Please click here for chordoma patient-related content; here, for chondrosarcoma patients.
Chordomas of the cranial base are commonly extradural, deriving from the clivus centrally, but rare intradural extension is possible. Chondrosarcomas originate along the paramedian central skull base from embryonic mesenchymal remnants and occupy the petrosphenoclival junction, with the majority centered at the temporo-occipital junction.
Both chordomas and chondrosarcomas are rare lesions and represent less than 1% of all intracranial tumors. Metastasis is possible and is associated with advanced local disease. The incidence of metastasis is variable, ranging from 4% to 43%, and generally affects the lymph nodes, axial skeleton, lungs, and skin. Metastatic lesions from chondrosarcomas are exceedingly rare.
Chordomas and chondrosarcomas have been difficult to differentiate due to their similar appearances on imaging. However, histopathologic distinctions facilitate their definitive diagnosis.
Chordomas are categorized into three different histologic types: classical low-grade, sarcomatous, and chondroid. These tumors stain positive for S-100, epithelial membrane antigen, cytokeratin, and brachyury. Histologically these tumors also demonstrate characteristic cells with large inclusion cysts, referred to as physaliferous cells.
Chondrosarcomas are negative for both epithelial membrane antigen and cytokeratin, making these a reliable method of distinction. These tumors are histopathologically classified into three types: classic, mesenchymal, and dedifferentiated. Both the mesenchymal and dedifferentiated classes are associated with a worse prognosis. They are also histologically graded as World Health Organization (WHO) grades 1, 2, or 3, with a correspondingly worse prognosis progressing from grades 1 to 3.
The complex overlying anatomy and close proximity to the brainstem, cranial nerves, and posterior circulation cerebrovasculature render effective resection of these tumors challenging. Chordoma and chondrosarcomas demonstrate a tendency to recur if gross total resection is not achieved, and therefore aggressive safe resection is advised.
Outcome data from the Surveillance, Epidemiology and End Results (SEER) database for patients with chordoma and chondrosarcomas demonstrated significantly worse outcomes for patients harboring chordomas. The median overall survival for patients suffering from chordomas was 12.6 years with a 10-year survival of 54.7%. Similar parameters for the patients with chondrosarcomas were 22 years and 68.2%, respectively.
Clinical Presentation
The most common initial presenting symptom is diplopia. This is most frequently attributed to cranial nerve VI palsy because of the proximity of the Dorello’s canal to the midline clivus. Headache is the second most common major presenting feature.
Visual field deficits, facial paresis, hearing impairment, vertigo, dysphagia, hoarseness, and palatal or tongue weakness are variably found at presentation. Less common features include facial numbness, facial pain, and pyramidal tract and cerebellar signs. Hydrocephalus may also occur with compression of the fourth ventricle.
Evaluation
The evaluation method of choice for a suspected skull base chordoma is magnetic resonance (MR) imaging and computed tomography (CT). Identification of cavernous sinus involvement requires particular attention to cranial nerves III-VI.
Chordomas and chondrosarcomas demonstrate an indistinguishable radiologic appearance on MR imaging. These lesions are seen as isodense/hypointense to gray matter on T1-weighted and hyperintense on T2-weighted sequences. They commonly demonstrate mild contrast enhancement. Chordomas may also demonstrate intralesional hemorrhage with residual hemosiderin or ferritin deposition. A high-resolution CT scan of the skull base characterizes the surrounding normal and affected bony anatomy, intralesional calcification, and any peripheral bony erosion. These tumors may also demonstrate encasement and potentially stenosis of the petrous internal carotid arteries.
Depending on the anticipated aggressiveness of planned surgical resection, a temporary balloon occlusion test of the ipsilateral internal carotid artery (ICA) may be performed to assess the ischemic risk if the artery is injured during the operation. Aggressive resection with bypass is not advised because complete removal of chordomas or chondrosarcomas is unlikely due to local infiltration and high likelihood of cavernous sinus invasion despite negative imaging findings of the sinus.
The distribution of the tumor within the sphenooccipital and petrosphenoclival regions determines the optimal surgical approach to maximize resection of chordomas and chondrosarcomas, respectively. The rostral half of the clivus is involved in 95% of cases, whereas the caudal clivus is involved in only 30%. The occipital condyles are affected in 30% of cases. Infiltration of the cavernous sinus is observed in 70% of patients with chordomas. More locally advanced lesions may demonstrate anterior extension involving the pterygopalatine fossa, paranasal sinuses, or the nasopharynx.
Multiple additional tumor types are commonly encountered within this skull base region. Therefore, chordomas and chondrosarcomas must be differentiated from other extradural masses that present with bone erosion along the clivus, including chondromyxoid fibroma, metastasis, meningioma, primary bone tumor, neurofibroma, neuroblastoma, lymphoma, hemangioma, and even fibrous dysplasia. Due to the heterogeneity of tumors in this area and lack of definitive differentiating features, biopsy of the mass before definitive resection is reasonable.
A baseline audiogram is necessary in patients whose cranial nerve VIII may be affected. Similarly, establishment of vision status with a formal vision field evaluation is advisable for patients with tumors impinging on the rostral cranial nerves. Anteriorly extending tumors can involve the retropharyngeal soft tissues; speech pathology evaluation for preoperative assessment of swallowing impairment is warranted. Extensive pharyngeal involvement poses a risk for airway stenosis and indicates the potential need for a postoperative tracheostomy.
Figure 1: A clival chordoma with intradural extension is shown. The mass is intermediate to low signal intensity on T1, carries small foci of hyperintensity (intratumoral hemorrhage or mucus pool), and is hyperintense on T2-weighted images. Note the heterogeneous enhancement pattern. CT discloses a destructive lytic lesion, sometimes with marginal sclerosis. The expansile mass is isodense or slightly hyperdense relative to the adjacent brain.
Figure 2: Chondrosarcomas demonstrate heterogeneous enhancement (arrow) and are hyperintense on T2-weighted images. In contrast to chordomas, chondrosarcomas have laterality and demonstrate less erosive margins on CT.
Treatment Paradigms
Management strategies for spheno-occipital chordomas and petrosphenoclival chondrosarcomas nearly universally include a surgical component. Surgery facilitates definitive diagnosis of the lesion and provides brainstem and cranial nerve decompression, and hence an opportunity for improvement in neurologic function. It also advances the safety of adjuvant radiotherapy by providing a more confined and focal target away from the vulnerable brainstem.
The management of these lesions may include observation, biopsy, surgical resection, radiotherapy, and their combinations. Observation should include serial imaging, but it is rarely the appropriate option if chordoma is in the differential diagnosis. Observation is generally considered more appropriate for low-grade biopsy-proven chondrosarcomas that are asymptomatic.
Biopsy is a reasonable consideration when surgical resection is contemplated and the diagnosis is uncertain. Based on the extradural location of the tumor, a biopsy can be easily performed through the nasal/oral pharynx or mastoid region. The characteristic paramedian location of chondrosarcomas, and often localized involvement to the petrous temporal bone, can make biopsy procedures challenging. On the other hand, chordomas frequently infiltrate into the retropharyngeal soft tissues that are readily accessible biopsy targets.
Radiotherapy alone for the treatment of chordomas and chondrosarcomas has limited support. Data from adjuvant use of radiotherapy suggest that small low-grade chondrosarcomas may be efficiently treated with radiotherapy alone, but further study is necessary to support this protocol. The major role of radiotherapy relates to prevention of recurrence after surgical resection. Radiotherapy modalities of choice include stereotactic radiosurgery, proton-beam radiotherapy, and intensity-modulated radiotherapy.
Chemotherapy has no proven role in the management of chordomas and chondrosarcomas. However, it may prove beneficial as a palliative measure for some patients with advanced or metastatic disease.
Because of the high recurrence rate of these tumors, the goal of surgery is gross total resection. This goal is unfortunately feasible in less than 30% of patients. At the time of presentation, most chordomas and chondrosarcomas are large (2-5 cm) and encompass multiple adjacent structures, including cerebrovasculature, cranial nerves, and brainstem. An alternative strategy is safe radical subtotal removal with planned postoperative radiotherapy.
In patients with advanced disease or recurrence, the identification of metastatic lesions is a contraindication to surgical resection. Involvement of our radiation oncology colleagues during the preoperative planning phase has proven useful to delineate the specific regions of the tumor best treated with surgery versus adjuvant radiotherapy. The goals of surgery and radiotherapy should be clearly defined before operative intervention.
Operative Anatomy
Chordomas commonly present at the midline, whereas chondrosarcomas originate along the paramedian central skull base. Therefore, an understanding of the intricacies of this bony anatomy is key to completing a successful operative session.
Chordomas are nearly universally centered over the clivus, and the clivus is almost always involved secondarily because chondrosarcomas extend medially.
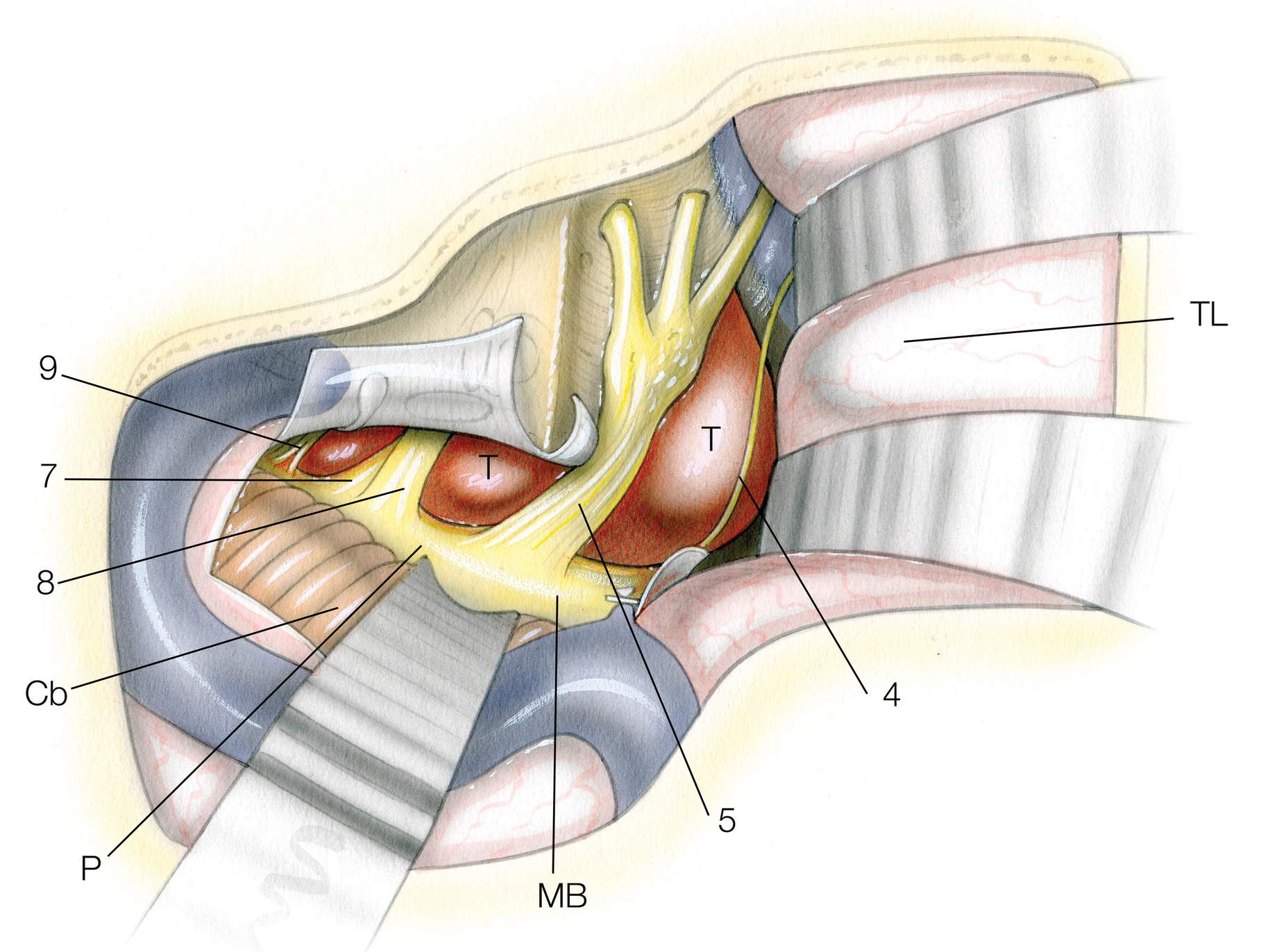
The clivus may be divided into three segments. The rostral third is defined as the region from the posterior clinoid processes and dorsum sellae to the petrous apices. The major neurovascular structures adjacent to this region include cranial nerves III, IV, V1, V2, and VI, cavernous ICA, upper basilar artery, and cavernous sinus. The middle third extends from the petrous apices adjacent to the Dorello’s canal to the jugular foramen (pars nervosa). The major related neurovascular structures include cranial nerves VII and VIII, lower basilar artery and vertebrobasilar anastomosis, and the inferior petrosal sinus. The caudal third extends from the jugular foramen (pars nervosa) to the foramen magnum. This segment contains the vertebral artery, occipital condyles, hypoglossal canal, and associated cranial nerves IX-XII.
The majority of chondrosarcomas extend medially into the adjacent petrous temporal bone. The apex of the petrous bone interfaces with the middle clival segment and the posterior margin of the greater sphenoid wing. The petrous segment of the ICA travels just anterior to the petrous apex across the foramen lacerum. Knowledge of the course of the petrous segment of the ICA is imperative during resection of tumors involving the petrous apex.
Click here to view the interactive module and related content for this image.
Figure 3: The osseous and neural anatomy near the upper clivus is shown. The dura over the right half of the floor of the clivus has been removed while preserving cranial nerves V-XII. The upper and middle clivus are divided by the dural entrance of the abducens nerve. The middle and lower clivus are separated by the jugular foramen. A red dotted line shows the demarcation between the upper and middle clivus, and a yellow dotted line shows the demarcation between the middle and lower clivus (upper row of images). Gruber’s ligament stretches from the lower part of the lateral edge of the dorsum sellae to the upper edge of the petrous apex. The Dorello’s canal (white dotted line) is bounded by Gruber’s ligament superiorly, the petrous apex inferolaterally, and the clivus inferomedially (middle row of images). The anatomy of the jugular foramen is shown (left lower image). The division between the upper and middle clivus corresponds to the upper edge of the petrous apex. The demarcation between the middle and lower clivus corresponds to the dural entry point of the glossopharyngeal nerve (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 4: Central endoscopic transnasal osteology of skull base anatomy is reviewed. The perpendicular plate of the ethmoid bone, vomer, and sphenoid crest make up the bony nasal septum. The sphenopalatine foramen is located just above the ethmoid crest in the lateral wall of the nasal cavity between the sphenoidal and orbital processes of the palatine bone (right upper image). The anatomy of the posterior wall of the sphenoid sinus is indicated. The Vidian canal is at the lower lateral corner of the sphenoid sinus (left middle image). The right lateral wall of the sphenoid sinus is shown with a 45-degree endoscope (left lower image). The anatomy of the posterior wall of the sphenoid sinus in another specimen is shown (right lower image)(images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 5: The right half of the lower clivus has been explored (top row of images). The vertical segment of the ICA along the lateral limit of the posterior wall of the sphenoid sinus is referred to as the paraclival segment. The lacerum segment of the petrous ICA is distal to the anterior genu of the artery. A thin dural bridge has been left behind at the junction of the upper and middle clivus (lower row of images) (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 6: The upper clivus has been disassembled in steps. The medial walls of the cavernous sinuses have been resected. Note the exposure of the relevant neurovascular structures via the transclival route. Many chondrosarcomas surround the paraclival and petrous ICA segments and extend into the cavernous sinus (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 7: An endoscopic view of the middle clivus is shown. A 45-degree endoscope is used to examine the foramen lacerum. The Vidian canal has been dissected (upper rows of images). A right lateral clivectomy was accomplished and the adjacent dura was removed. A dural bridge demonstrates the borders between the upper and middle clivus, and a thin bony bridge separates the middle and lower clivus (middle row of images). A closer view using a 45-degree endoscope directed laterally is also shown (left lower image). The petrous apex and the bone below the distal carotid canal have been removed to expose the cisternal segments of the cranial nerve VII/VIII complex (right lower image) (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 8: A right lower clivectomy is demonstrated (left upper image). Note the anatomy of the lower cranial nerves. A bilateral lower clivectomy is also shown (right upper image). The anterior surface of the medulla and the vertebral, posterior inferior cerebellar, and anterior spinal arteries are evident (upper row of images). Midsagittal sections of other specimens show the relationship between the posterior nasopharynx, its associated muscles, and the lower clivus (lower row of images) (images courtesy of AL Rhoton, Jr).
SURGICAL RESECTION OF CHORDOMA AND CHONDROSARCOMA
Surgical Approaches
The deep anatomy of the central skull base permits designation of multiple approaches to a given anatomic region; therefore, the operative corridor to each chordoma or chondrosarcoma must be individualized. Collaboration between the neurosurgeon and the endoscopic rhinologist assists in selection of the optimal surgical approach for each patient’s tumor. The major factors to consider when determining the optimal surgical approach are the size of the tumor, as well as its origin, direction of extension (long axis), abutment or encasement of neurovascular structures, degree of bone erosion, and the expertise of the participating surgeons. The radiation oncologist directs the surgical team regarding tumor locations that cannot be effectively treated via radiotherapy because of the proximity of the brainstem.
The transnasal endoscopic routes are ideal for reaching chordomas and chondrosarcomas because these tumors are extradural and unlikely to be fibrous; they are amenable to removal using angled endoscopes and limited microsurgical handling. In addition, minimally disruptive transcranial routes to the midline skull base are not feasible.
These surgical maneuvers can remain extradural, except rarely when intradural extension is encountered. Intradural resection is followed by dural reconstruction.
Chordomas and chondrosarcomas can readily infiltrate the cavernous sinus despite unremarkable imaging findings in this location. This involvement significantly limits gross total removal of these lesions; therefore, aggressive resection paradigms that can place nearby neurovascular structures at risk are not advised.
Table 1 summarizes my preferences for reaching paraclival tumors. Please note that I have abandoned the use of most approaches to these tumors in favor of the endoscopic transnasal paraclival route.
| Approach | Paraclival Region |
| Endonasal | |
|
Endoscopic endonasal transclival/paraclival |
Rostral, middle and caudal third |
| Transcranial | |
|
Bilateral subfrontal |
Rostral third |
|
Frontotemporal transcavernous |
Rostral third |
|
Anterior petrosectomy transcavernous |
Rostral third |
|
Posterior petrosectomy transcavernous |
Middle third |
|
Transcondylar |
Caudal third |
| Other | |
|
Anterior transfacial |
All |
Endoscopic Transnasal Transclival Approach
I use intraoperative image guidance based on a high-resolution CT angiogram to localize the tumor and the osteovascular anatomy. Localization of the paraclival ICAs is imperative to keep them out of harm’s way.
Once the nasoseptal flap is elevated and the clivus is exposed, a diamond burr is used to drill the clivus/petroclival junction overlying the bulk of the tumor. The limits of bone removal are the floor of the sella superiorly, the foramen magnum inferiorly, and the ICAs and occipital condyles laterally.
The bone over the tumor is attenuated and is an appropriate starting point for drilling. Unroofing the center of the mass allows the ring curettes to evacuate these soft tumors. The initial tumor decompression guides the next steps in drilling to uncover the margins of the mass.
Most chondrosarcomas encase the petrous ICA, but do not invade its arterial wall. Upon removal of the tumor components around the ICA and its identification, this artery is further unroofed using Kerrison rongeurs. Recognition of its route keeps the artery out of harm’s way. The angled ring curettes and endoscopes afford panoramic visualization around the skeletonized ICA, delivering the tumor from the space along the posterolateral aspect of the artery within the operative blind spot.
The dura of the posterior fossa is protected during handling of the tumors that respect their dural borders. Intradural invasion of the tumor provides a transdural trajectory for removal of additional tumor fragments. I follow the path of the tumor around the ICA into the cavernous sinus and remove deliverable tumor portions. Bleeding from the cavernous sinus is controlled using Floseal hemostatic matrix (Baxter, Deerfield, IL) packing. After tumor removal, the transdural corridor is sealed with stripes of fat, covered with a piece of allograft dura, and the entire operative space covered by the nasoseptal flap.
Angled endoscopes and advanced experience with the use of complex skull base endoscopic techniques provide an unparalleled opportunity to the surgeon to achieve maximal resection of these deep-seated tumors with minimal risk to the patient.
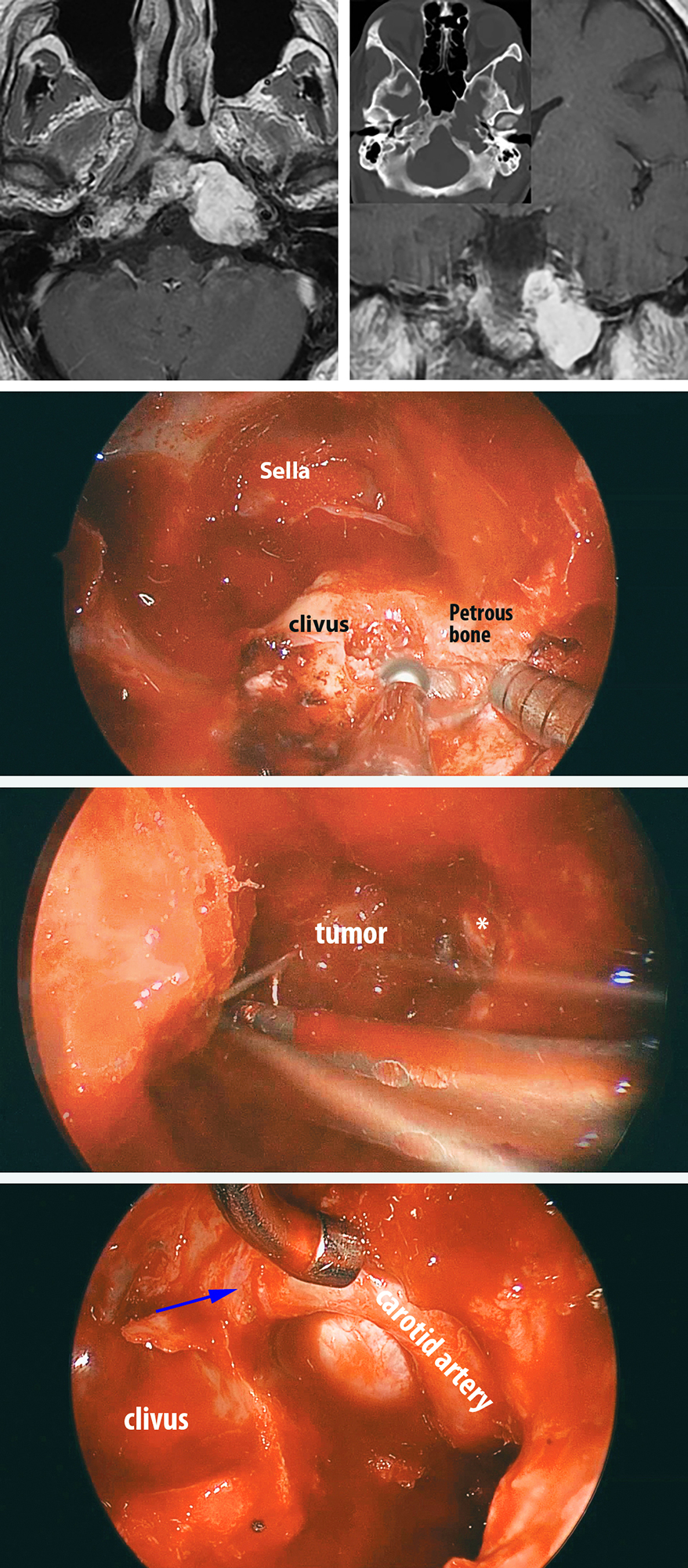
Figure 9: This illustration with its layered format provides a road map for surgery within the periclival region. Note the anatomical relationships of the abducens nerve to the clival dura and carotid artery. The location of the tumor within the retrocarotid space complicates its exposure. Note the relationship of the vidian canal to the foramen lacerum. Skeletonization of the paraclival artery is necessary for most sphenopetroclival chondrosarcomas/chordomas. Note the correlation of the bone to the underlying cerebrovascular structures.
Figure 10: Pterygosphenoid fissure is a reasonable landmark for the foramen lacerum and the start of drilling. The carotid artery is exposed early and unroofed. Early identification of the artery will avoid its subsequent inadvertent injury. The paraclival artery may be displaced by the tumor but the location of the artery within the foramen is constant. The tumor is also uncovered. Navigation via a thin sliced CT scan is reasonable to localize the bulk of the tumor and the route of the artery. The "discolored bone" signifies the typical location of the tumor.
Figure 11: This image demonstrates the operative field as the tumor is being evacuated via ring curettes. The soft tumor is removed under the direction of angled endoscopes. Stimulation mapping of the abducens nerve may be necessary to avoid its injury. Its expected route is shown. The carotid artery is unroofed to the level of the cavernous sinus so that the artery can be gently mobilized laterally so that the retrocarotid space is reachable. The infiltrated soft bone is also removed via bone curettes and drilling. The dura is typically intact and should not be violated unless the preoperative imaging warrants its opening.
Figure 12: The above anatomical relationships are further explored in the following left-sided stepwise cadaveric dissections (Images courtesy of Juan Fernandez-Miranda).
For more details on this approach, refer to the Endoscopic Expanded Transnasal Approach chapter. Cholesterol granulomas and mucoceles are approached via a similar operative trajectory.
Figure 13: A left petroclival chondrosarcoma (top images) underwent removal via the endoscopic transnasal transpetroclival route. The relevant operative anatomy is marked in the photo in the middle row. Angled ring curettes and endoscopes afforded removal of the tumor hidden around the medial petrous ICA (image in the third row). A final view of the operative field is shown in the bottom image. Note the skeletonization of the petrous ICA to the level of its entry into the cavernous sinus (blue arrow). Such an operative reach would be arduous via the transcranial corridors.
Nonendoscopic Anterior Transfacial Approach
The nonendoscopic anterior transfacial approach provides direct and wide clival exposure. This approach can furnish customized access via the extended transsphenoidal, transethmoidal, transclival, transmaxillary, or transoral components.
The exposure readily permits resection of rostral, middle, and caudal clival lesions. Pitfalls to the “open” transfacial approach include its significant extent of disruption of the normal anterior facial anatomy, high risk for cerebrospinal fluid (CSF) leakage, and restriction in lateral dissection within the paraclival posterior fossa.
Bilateral Subfrontal Approach
The bilateral subfrontal approach provides wide access to the rostral clival segments, with more extensive lateral exposure than those afforded via the endoscopic endonasal transclival approach.
The extent of lateral dissection through the microscopic visualization beyond the carotid arteries and petrous apices may be expanded via the use of an angled endoscope. The pitfalls of this approach are its invasive nature and the limitations associated with its inferior reach. For additional discussion about this approach, see the Bifrontal Craniotomy chapter.
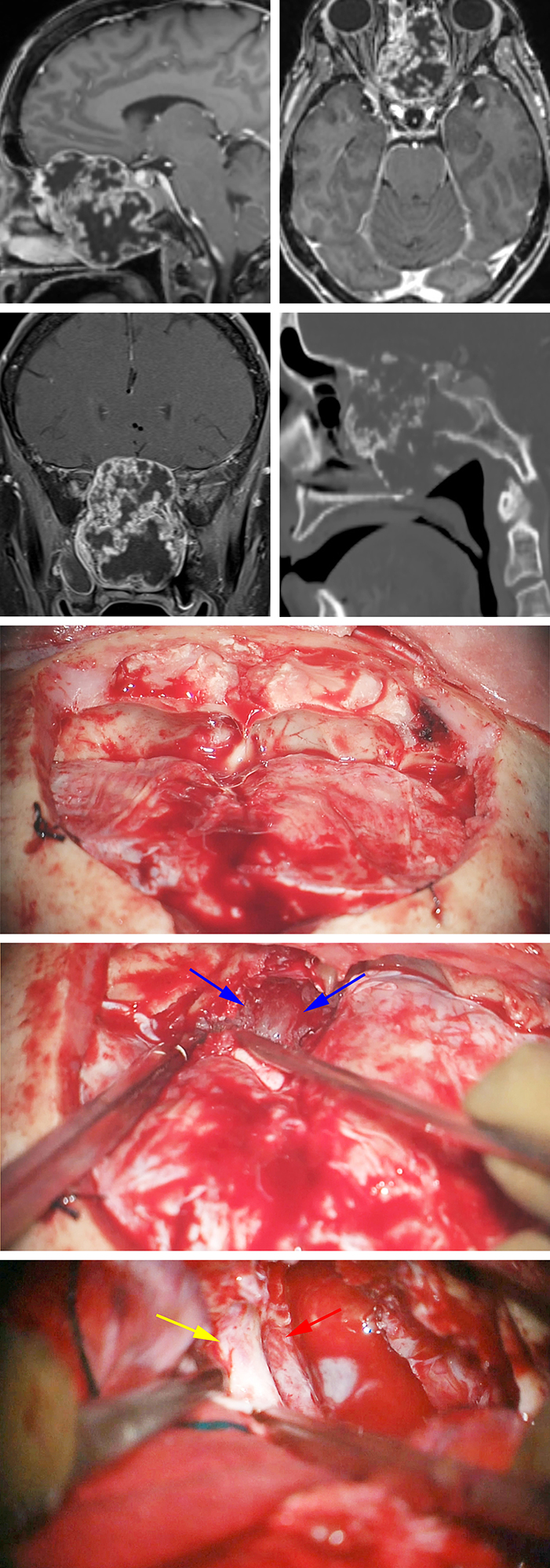
Figure 14: A large anterior skull base chondrosarcoma is evident. Note the erosive pattern of bone changes on the sagittal CT scan (upper images). This tumor was resected via combined bifrontal (middle photos) and endoscopic transnasal routes. The tumor eroding through the cribriform plate is marked with blue arrows (second intraoperative photo). Bilateral optic nerves (left nerve covered by its dura, yellow arrow) and ICAs (red arrow) were exposed via the bifrontal route at the end of the resection (lower photo).
Anterior Cranial Base Chondrosarcoma: Bifrontal Approach
Frontotemporal Transcavernous Approach
The frontotemporal transcavernous approach is optimized for accessing tumors lateralizing to one side, harboring both intradural and dominant resectable cavernous sinus involvement. The craniotomy requires a combination of a frontotemporal craniotomy, orbitozygomatic osteotomy, and extradural clinoidectomy.
Dissection of tumor within the cavernous sinus demands vascular control over the carotid artery along its cavernous and clinoidal segments to manage inadvertent bleeding from the vessel during dissection of any tumor fragments that may have invaded the arterial wall.
Proximal ICA control is achieved through a small skin incision in the patient’s neck in select cases. I do not believe the risks of a high-flow revascularization procedure and cranial nerve injury justify the goal of achieving a more radical subtotal tumor removal.
This approach does not provide sufficient exposure or flexible working angles to tackle tumors with significant bone involvement. This restriction can lead to a higher risk of recurrence; therefore, this approach may be combined with other routes (endonasal) to maximize resection of bone infiltration.
Anterior Petrosectomy Approach
The anterior petrosectomy approach is favored for resection of tumors and, more specifically, chondrosarcomas that involve the upper clivus and petrous bone. The laterality of chondrosarcomas and their involvement with the petrosphenoclival junction make this approach desirable for this tumor subtype. The limitations in the inferior and contralateral midline access are noteworthy and should be considered when assessing the applicability of this approach. This method provides access to the middle fossa in addition to the petrous apex and rostral clivus.
This pathway is described in more detail in the Anterior Petrosectomy chapter.
Following a standard anterior petrosectomy, the tumor can be decompressed using ring curettes. The characteristic soft and suckable texture of chordomas and chondrosarcomas significantly facilitates their removal via limited operative corridors. After evacuation of the soft part of the tumor, drilling can excise the affected bony margins as guided by intraoperative image guidance based on CT navigation. Endoscopic-assisted microsurgery can improve the extent of resection.
For tumors located along the medial and inferior aspect of the petroclival junction, the ICA is mobilized out of its canal so that drilling can expose the petroclival synchondrosis. This process begins with identification of the petrous segment at the anteromedial middle fossa floor by following V3 to its junction with the greater superficial petrosal nerve.
Next, the dissection proceeds along the distal ICA to complete skeletonization of the artery along its horizontal petrous segment to the point where it interfaces with the cavernous sinus. To complete the exposure of the petroclival synchondrosis, I rotate the petrous ICA anteriorly to facilitate drilling the bone along the medial carotid wall. The clivus can then be drilled further away from the petrous apex toward the foramen magnum, depending on the extent of the tumor. The trochlear nerve should be found and preserved when possible.
The procedure can include cavernous sinus exploration. Only safe tumor debulking without manipulation of the functional cranial nerves is recommended. Essentially, the path of the tumor is pursued into the cavernous sinus and the tumor is gently evacuated. Additional details can be found in the Cavernous Sinus Meningioma chapter.
The pitfalls of this approach include the risk of CSF leakage due to the proximity of this trajectory to the Eustachian tube. Intraoperative identification of the Eustachian tube and its prophylactic packing, when necessary, with a fat graft, avoids persistent CSF leakage. CSF leakage can also be caused by drilling the aerated clivus.
Posterior Petrosectomy Approach
The posterior petrosectomy approach affords exposure of a lesion located more caudally along the clivus (middle third). For a detailed description of this osteotomy, see the Extended Posterior Petrosectomy chapter.
This approach includes the retrolabyrinthine, translabyrinthine, and transcochlear variants, which enhance accessibility to the lateral, anterolateral, and anterior brainstem territories, respectively. These approaches provide a progressively wider visualization of the presigmoid posterior fossa. The obvious pitfalls to the use of this approach are the loss in hearing and potentially facial function associated with the use of the more radical osteotomy variants.
Transcondylar Approach
The transcondylar approach is indicated for optimal access to caudal clivus, occipital condyle, and anterolateral foramen magnum lesions. A complete description of the transcondylar approach is included in the Transcondylar Approach chapter. The C1 lateral mass may be removed to optimize access to the caudal clivus.
The pitfalls of this approach include the potential need for an occipitocervical fusion, if greater than 50% of the tumor-affected occipital condyle is removed or if significant ligamentous disruption occurs during the approach.
Postoperative Considerations
The surgeon should watch for CSF leakage following procedures that involve drilling the mastoid, sphenoid, clivus or petrous bone. This risk can be minimized by intraoperative identification of the violated air cells, repeatedly waxing the cells, and performing a two-layered closure with globules of fat and pericranium. Potential empty paradural spaces should be filled with fat.
Following the postoperative suspicion of a CSF leakage, a high-resolution (thin-slice) CT scan of the skull base can help identify the exact site of the leak. Temporary lumbar CSF drainage via a lumbar drain or repeated spinal taps can decrease the flow of CSF across the persistent fistula. Surgical exploration may be required if the tract is refractory to closure upon lumbar drainage.
Transient neurologic deficits are expected if the cranial nerves are manipulated during surgery. Permanent cranial nerve deficits are the result of surgical trauma or ischemic injury.
Pearls and Pitfalls
- The optimal surgical trajectories to reach chordomas or chondrosarcomas should include the liberal use of skull base osteotomies instead of parenchymal retraction. The endoscopic transnasal routes are the most flexible routes to access these tumors.
Contributor: Benjamin K. Hendricks, MD
For additional illustrations of anterior approaches to the clivus, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of lateral approaches to the clivus, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of using endoscopes during skull base surgery, please refer to the Jackler Atlas by clicking on the image below:
References
Jones PS, Aghi MK, Muzikansky A, Shih HA, Barker FG. Outcomes and patterns of care in adult skull base chondrosarcomas from the SEER database. J Clin Neurosci. 2014;21:1497-1502.
Jones PS, Aghi MK, Muzikansky A, Shih HA, Barker FG, Curry WT. Outcomes and patterns of care in adult skull base chordomas from the Surveillance, Epidemiology, and End Results (SEER) database. J Clin Neurosci. 2014;21:1490–1496.
Nader R, Gragnaniello C, Berta SC, Sabbagh AJ, Levy ML (eds): Procedure No. 52, in: Neurosurgery Tricks of the Trade—Cranial. New York, Stuttgart, Delhi, Rio: Thieme Medical Publishers, 2014.
Raper DMS, Komotar RJ, Fraser JF, Anand VK, Moore N, Schwartz TH. Skull base chordomas: endonasal endoscopic transclival approach, in Hayat MA (ed): Tumors of the Central Nervous System, Volume 8. Netherlands: Springer, 2012.
Rhoton LA, Seoane E. Surgical anatomy of the skull base, in Harsh GR, Janecka IP, Mankin HJ, Ojemann RG, Suit H (eds): Chordoma and Chondrosarcomas of the Skull Base and Spine. New York: Thieme Medical Publishers, 2003.
Rostomily RC, Sekhar LN, Elahi F. Chordomas and chondrosarcomas, in Sekhar LN, Fessler RG (eds): Atlas of Neurosurgical Techniques: Brain. New York: Thieme Medical Publishers, 2006.
Sen CN, Sekhar LN, Schramm VL, Janecka IP. Chordomas and chondrosarcomas of the cranial base. Neurosurgery. 1989:25:931–941.
Sim H, Frassica FJ, Wold LE, McLeod RA. Chondrosarcoma of the spine: the Mayo Clinic, in Sundaresan N, Schmidek HH, Schiller AL, Rosenthal DI (eds): Tumors of the Spine. Philadelphia: WB Saunders, 1990:155–162.
Van Gompel JJ, Janus JR. Chordoma and chondrosarcoma. Otolaryngol Clin N Am. 2015;48:501–514.
Volpe R, Mazabraud A. A clinicopathologic review of 25 cases of chordomas (a pleomorphic and metastasizing neoplasm). Am J Surg Pathol. 1983;7:161–170.
Weber AL, Brown EW, Hug EB, Liebsch NJ. Cartilaginous tumors and chordomas of the cranial base. Otolaryngol Clin North Am. 1995;28:453–471.
Please login to post a comment.