Cerebellar AVMs
This is a preview. Check to see if you have access to the full video. Check access
Petrosal AVM
Please note the relevant information for patients suffering from arteriovenous malformations is presented in another chapter. Please click here for patient-related content.
Cerebellar arteriovenous malformations (AVMs) make up 10% to 14% of all AVMs and are more likely to present with hemorrhage and inflict neurologic deficits than their cerebral counterparts. Because of their aggressive course, a lower threshold for pursuing treatment of unruptured lesions is reasonable. These AVMs are readily exposed via traditional suboccipital, retromastoid, and supracerebellar craniotomies and their combinations.
Operative Anatomy
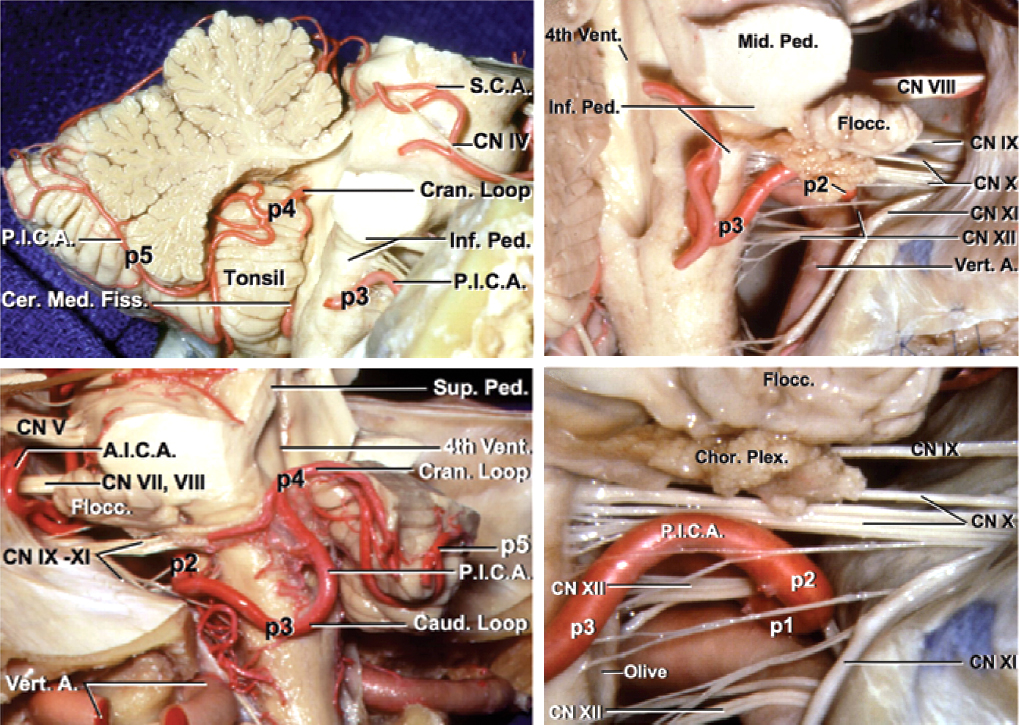
Each hemisphere of the cerebellum has tentorial, petrosal, and suboccipital surfaces, and each surface has its associated fissure that divides the hemisphere into lobules. The falx cerebelli runs between the two hemispheres and forms the posterior cerebellar incisura adjacent to the vermis. Inferiorly, the cerebellar tonsils extend into the cisterna magna, separated in the midline by the vallecula. The opening into the fourth ventricle via the foramen of Magendie is deep to the vallecula.
Figure 1: The tentorial (top), suboccipital (middle), and petrosal (bottom) surfaces of the cerebellum and their relevant anatomy are shown. The names of these surfaces are based on their overlying structures (images courtesy of AL Rhoton, Jr).
Cortical branches of the superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), and posterior inferior cerebellar artery (PICA) form the main arterial supply to the cerebellum and participate in AVMs of the region.
The SCA comprises four segments:
- Anterior pontomesencephalic segment (S1): from the SCA origin to the anterolateral brainstem coursing beneath cranial nerve (CN) III
- Lateral pontomesencephalic segment (S2): from the anterolateral brainstem caudally to the trigeminal root until it enters the cerebellomesencephalic fissure
- Cerebellomesencephalic segment (S3): within the cerebellomesencephalic fissure and eventually accompanying CN IV through hairpin curves and reaching the tentorial edge
- Cortical segment (S4): emerging from the cerebellomesencephalic fissure and supplying the tentorial surface of the cerebellum. This segment bifurcates into the rostral and caudal trunks, supplying the superior vermis and hemispheric surface, respectively. These trunks also give rise to smaller precerebellar arteries supplying the deep cerebellar nuclei, and are particularly important as they become the deep perforating arterial supply to cerebellar AVMs.
Click here to view the interactive module and related content for this image.
Figure 2: The relevant segmental anatomy of the SCA is shown (images courtesy of AL Rhoton, Jr). Early identification and occlusion of the AVM-associated arteries arising from this vascular tree is part the key maneuver to tackle tentorial and cerebellopontine (CP) angle AVMs.
The AICA also comprises four segments:
- Anterior pontine segment (A1): from the origin of the AICA, proximal to CN VI root exit zone, and ending along the long axis of the inferior olive
- Lateral pontine segment (A2): from the anterolateral margin of the pons through the CP angle up to the flocculus, with terminal branches including the labyrinthine artery, recurrent perforating artery, and subarcuate artery
- Flocculopeduncular segment (A3): from the flocculus to the cerebellopontine fissure, commonly with rostral and caudal trunks surrounding CNs VII/VIII
- Cortical segment (A4): distal to the cerebellopontine fissure to supply the cerebellar petrosal surface
Click here to view the interactive module and related content for this image.
Figure 3: The relevant segmental anatomy of the AICA is shown (images courtesy of AL Rhoton, Jr).
The PICA is divided into five segments:
- Anterior medullary segment (P1): the origin of the PICA courses anterior to the medulla and passes around the hypoglossal rootlets, terminating at the medial border of the inferior olive.
- Lateral medullary segment (P2): this short segment has its origin at the most prominent point of the inferior olive to the rootlets of CNs IX/X/XI at the lateral edge of the olive.
- Tonsillomedullary segment (P3): descends from the lateral edge of the olive to the inferior pole of the cerebellar tonsil and reverses rostrally along the medial tonsil (the infratonsillar/caudal loop).
- Telovelotonsillar segment (P4): ascends from the midpoint of the cerebellar tonsil toward the roof of the fourth ventricle and turns caudally, coursing posteriorly toward the tonsillobiventral fissure (the supratonsillar/rostral loop). This branch perfuses the choroid plexus of the fourth ventricle and the inferior medullary velum.
- Cortical segment (P5): its origin emerges from the tonsillobiventral fissure with two medial and lateral trunks for the inferior vermis and tonsil/cerebellar hemisphere, respectively. Superior tonsillar branches can be a source of deep perforating arterial supply to large AVMs.
Click here to view the interactive module and related content for this image.
Figure 4: The relevant segmental anatomy of the PICA is shown (images courtesy of AL Rhoton, Jr).
The venous anastomotic configurations for the cerebellum may be divided into three groups.
Superficial venous supply:
- Tentorial surface: Superior hemispheric and superior vermian veins
- Suboccipital surface: Inferior hemispheric and inferior vermian veins
- Tonsils: Medial and lateral tonsillar and retrotonsillar veins
- Petrosal surface: Anterior hemispheric veins
Deep venous supply:
- Vein of cerebellomesencephalic fissure: Travels adjacent to the superior half of the fourth ventricular roof
- Vein of cerebellomedullary fissure: Courses adjacent to the inferior half of the fourth ventricular roof
- Vein of cerebellopontine fissure: located adjacent to the lateral recess
Bridging veins:
- Galenic group: Drain the superior surface of the cerebellum within the midline and join the vein of Galen
- Tentorial group: Drain the tentorial cerebellar surface and join the torcular Herophili or tentorial sinuses
- Petrosal group: Drain the petrosal cerebellar surface and join the superior and inferior petrosal sinuses
The cortical segments of the arteries listed above are most involved with sustaining arteriovenous shunting for cerebellar AVMs. These segments include the s4 SCA and the a4 AICA, as well as the p4 and p5 PICA. The s3 and s4 distal branches (rostral trunk) give rise to some of the daunting deep white matter feeders which cause substantial technical challenges regarding their control during removal of large and deeply rooted AVMs.
CEREBELLAR ARTERIOVENOUS MALFORMATION RESECTION
The complexity of the microsurgery for managing cerebellar AVMs stems from the limited space of the posterior fossa and the vulnerability of the nearby brainstem and cranial nerves. Direct involvement of the eloquent neural structures is observed only if the cerebellar AVM infiltrates or reaches the deep cerebellar nuclei (seen only with the suboccipital subtype). Cerebellar AVMs may be categorized into five subtypes based on their anatomic distribution.
I find managing the deep white matter feeders of large cerebellar AVMs more challenging than the cerebral ones. The technical difficulty is most likely related to the abundant white matter arterial plexus normally present in the cerebellum.
Tentorial AVMs
Tentorial AVMs are unilateral entities that receive their blood supply primarily from the hemispheric branches of the SCA. These branches commonly join the nidus along its anterior and medial margins. Venous drainage favors the superficial hemispheric veins anastomosing with the vein of Galen anteriorly and the torcula posteriorly.
These lesions are approached via the paramedian supracerebellar infratentorial craniotomy. The patient is placed in the lateral position and the patient’s neck is flexed to facilitate an increased exposure of the tentorial surface. A lumbar drain is installed for early cerebrospinal fluid (CSF) drainage during supracerebellar dissection, obviating the need for removal of the bone over the cisterna magna. This approach unroofs the transverse sinus, mobilizes the sinus with the elevation of the dural flap, and provides direct access to the supracerebellar corridor.
Upon disclosure of the AVM, I inspect the superficial borders of the nidus and the arterialized draining hemispheric veins; nonarterialized bridging veins may need to be sacrificed for adequate supracerebellar working space. The nidal dissection is carried out around the draining vein and the cortical SCA branches are interrupted. A hematoma forms an ideal cleavage plane for this step of dissection.
As the nidus is circumdissected further, it is mobilized superiorly to allow identification of the deeper arterial feeders. As these feeders are disrupted, the draining vein turns dark blue. Once all arterial feeders are excluded, the main draining vein is sacrificed and the nidus is extracted. Care should be taken to prevent thermal or traction injury to the vulnerable CN IV that courses within the dural leaflets of the tentorial incisura.
Figure 5: The surgeon’s view (top) of a classic tentorial AVM is demonstrated. Note the supplying arteries and draining veins. An additional view for identification of the angioarchitecture is shown in the second sketch. The bridging AVM-associated draining veins should be preserved during superacerebellar dissection. Some of these veins join the vein of Galen by travelling over the cerebellum.
Figure 6: A large tentorial AVM and the associated intraoperative findings are included. The first two rows demonstrate the angioarchitecture of the malformation. The patient was placed in the lateral position and a generous unilateral suboccipital craniotomy was completed. The large hemispheric arterialized draining vein was identified and protected (second row). The SCA feedings vessels (arrow), just behind the draining vein, were identified and coagulated; the nidus was then circumferentially isolated (fourth row). Following disconnection of the nidus, the draining vein appeared dark and therefore was sacrificed. Entry into the fourth ventricle assured complete removal of the periventricular portion of the malformation (last row).
Tentorial AVM: Principles of Resection
Suboccipital AVMs
Suboccipital AVMs are also unilateral entities, receiving blood supply from the cortical branches of SCA (s4) superiorly, AICA (a4) laterally, and PICA (p5) inferiorly. Venous drainage occurs through superficial hemispheric or vermian veins, anastomosing with the torcula and transverse sinus.
I use a standard unilateral suboccipital craniotomy to approach suboccipital AVMs. Larger lesions with lateral or superior extension may be addressed via a combination of suboccipital and retrosigmoid or supracerebellar approaches, respectively.
I also prefer to place the patient in the lateral position with the neck slightly flexed. This setup allows me to sit during the microsurgical part of the operation that can be lengthy. It also uses the gravity to drain blood and fluids out of my operative field. A linear paramedian or a hockey stick incision is followed by the craniotomy that crosses the midline. The dura is opened in a cruciate manner. The cisterna magna is opened to drain additional CSF (a lumbar drain is also used) and provide cerebellar relaxation.
Suboccipital AVMs and their venous drainage are superficial and readily discoverable after dural opening. The hemispheric and vermian veins are identified, laterally and medially, respectively. The above-mentioned arterial supply branches are coagulated sequentially and divided.
Eloquence is not a concern with suboccipital AVMs unless extension of the deep white matter feeders into the deep nuclei is present. Once the superficial arteries are disrupted and parenchymal circumdissection is pursued around the lesion, the nidus is mobilized and the deeper feeders are handled.
Figure 7: The angioarchitecture of suboccipital AVMs is demonstrated. The surgeon’s view highlights the importance of early disconnection of the feeding arteries on the cortical surface (top image). The deep white matter feeders can be quite robust and problematic in this AVM class (bottom photo). These feeders can extend to the level of the fourth ventricle and approach the deep nuclei (inset).
Tonsillar AVMs
Tonsillar AVMs are the least common of the cerebellar AVM subtypes and are localized to the caudal region of the suboccipital surface. These lesions are unilateral and primarily perfused by the ipsilateral PICA. Venous drainage recruits the medial/lateral tonsillar and retrotonsillar veins.
A standard unilateral suboccipital craniotomy using a paramedian or hockey stick incision easily exposes these lesions. Some of the more caudal lesions benefit from unroofing of the foramen magnum and a C1 laminectomy to optimize exposure.
The proximal PICA is identified under the tonsil and pursued distally to where it often ends in the AVM. All of the en passage vessels arising from the more proximal segments of the PICA are protected. Standard circumferential nidal dissection mobilizes the lesion superiorly and allows more optimal control over the p4/p5 feeding branches of the PICA. Eloquence is not a concern, but thermal injury to the medulla, CNs IX, X, XI, and the PICA must be avoided.
Figure 8: The angioarchitecture of tonsillar AVMs is illustrated. Note the contributions of the PICA branches.
Petrosal AVMs
Petrosal AVMs are unilateral lesions seizing arterial supply via the AICA branches. Venous drainage occurs via the anterior hemispheric vein and the vein of the cerebellopontine fissure anastomosing with the superior petrosal sinus. These AVMs are unique in their anatomic relation relative to the pontomedullary junction, specifically their proximity to CN V and the CN VII/VIII complex.
Figure 9: The anatomy of AVMs on the petrosal surface is highly variable relative to the brainstem and cranial nerves. Note the feeding arteries from the AICA and SCA and the draining veins joining the superior petrosal sinus.
An extended retrosigmoid approach is ideal for revealing these lesions. The patient is placed in the park-bench position; however, a larger body habitus demands the lateral body position to avoid excessive neck torsion. Brainstem auditory evoked responses (BAERs) are monitored intraoperatively. The craniotomy uncovers the transverse-sigmoid junction and unroofs the sigmoid sinus. The dural flap is incised along the dural sinuses.
The arachnoid membranes over the CP angle cisterns are dissected so that the anterior margin of the nidus is first exposed and additional CSF is released. Next, the brainstem and cranial nerves are localized relative to the anterior margin of the lesion so they are not in harm’s way during AVM resection.
The arterial feeders are identified rostrally at the level of CN V where SCA branches feed the AVM, and caudally at the level of CNs IX, X, and XI where the AICA and PICA branches supply the nidus. The deep planes of dissection around the AVM require some cerebellar resection lateral to the nidus so that the medial margin of the nidus is reached via anterior mobilization of the lesion away from the middle cerebellar peduncle. These maneuvers provide adequate visualization of the deep AICA feeders arising from the a3 and a4 segments. These vessels can then be divided to complete circumdissection of the nidus.
Despite the noneloquent location of the petrosal AVMs, neural structures are at risk at the anterior edge of the operative field. Consequently, it is important to understand the anatomic proximity of the pons, middle cerebellar peduncle, and CNs V/VII/VIII complex.
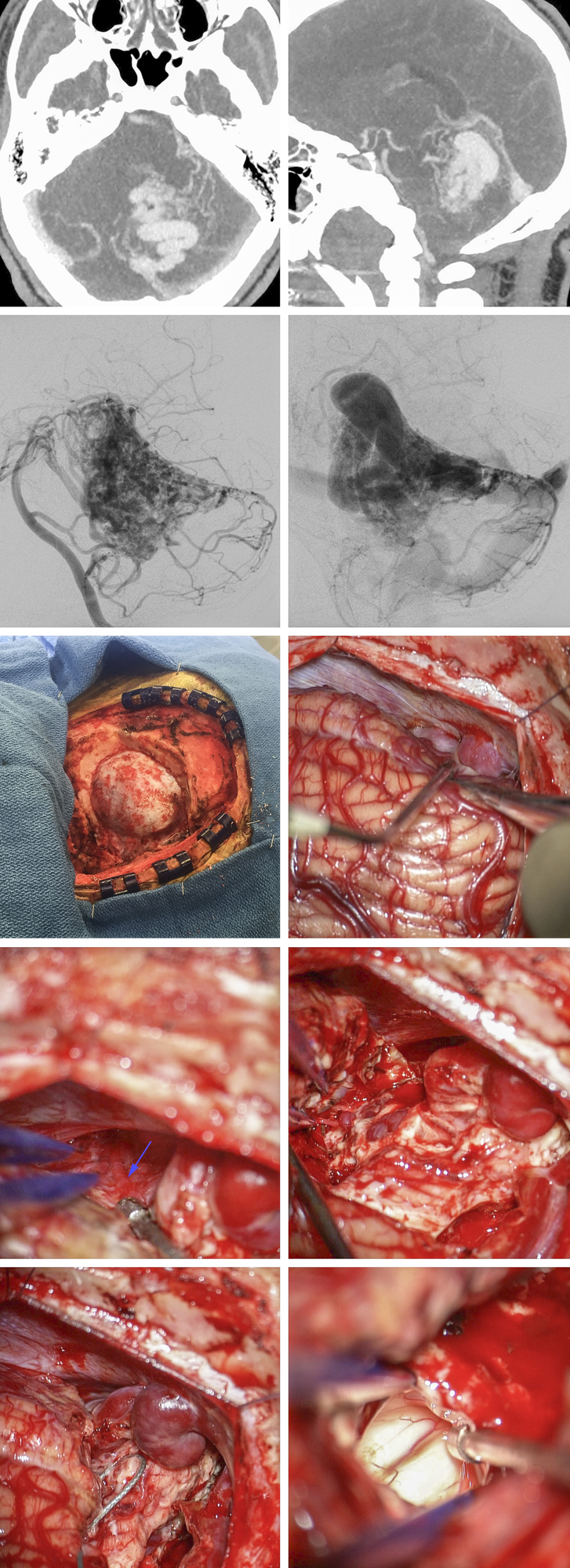
Figure 10: A 65 year-old male presented with an spontaneous cerebellar ICH and was diagnosed with a small petrosal AVM fed by the superior (SCA) and anterior inferior cerebellar arteries (AICA)(top row). Left-sided retromastoid approach exposed an arterialized superior petrosal vein (purple arrow) and a normal appearing vein (blue arrow) near the trigeminal nerve (V). A feeding artery arising from the AICA is also shown (red arrow)(second row, left image). The AVM was circumferentially isolated laterally (second row, right image). Other feeding vessels from the AICA were found at the lower pole of the AVM and disconnected (third row). Finally, the SCA feeding vessels were also sacrificed and the nidus was extracted (last row).
Vermian AVMs
Vermian AVMs are the most common cerebellar AVM subtype, with the majority of them occupying the superior vermis. Superior vermian lesions receive bilateral arterial supply from the SCA branches. On the other hand, the inferior vermian lesions feed from the bilateral PICA branches. As would be expected, the venous drainage patterns differ for these two classes of lesions; the superior ones drain into the superior vermian vein connecting with the vein of Galen, whereas the inferior lesions drain into the inferior vermian vein anastomosing with the torcula.
Figure 11: The pathoanatomy of a typical vermian AVM is noted. The SCA feeding vessels and drainage system are shown (surgeon's view, top image). A sagittal perspective of the pathology is also included for further demonstration of a non-operative view.
Vermian AVMs are exposed through a tailored suboccipital craniotomy with the addition of a supracerebellar osteotomy for superior vermian lesions. The patient is placed in the lateral or park-bench position while the neck is flexed to orient the surface of the tentorium approximately perpendicular to the midcoronal plane of the patient’s body.
The sitting or concorde position may also be used for patients with superior vermian lesions. A lumbar drain is placed preoperatively for CSF drainage and brain relaxation, avoiding cerebellar herniation upon dural opening. The necessary planes of dissection differ based on the rostral-caudal location of the AVM.
A unique feature of this type of midline AVM is that it recruits bilateral arterial feeders and therefore requires a bilateral suboccipital craniotomy. This feature has practical importance during surgical dissection as I search for feeding vessels on both sides of the vermis.
Because these AVMs can reach a large size, I sometimes resect a small section of the posterior vermis overlying the borders of the nidus in order to gain exposure for mobilization of the nidus and disconnection of its deep feeders. This measure is particularly necessary for deep-seated vermian AVMs.
The en passage proximal branches of the s4 SCA segment that supply the tectum and posterior midbrain should be protected. The risk of cerebellar mutism is minimized through a conservative vermian transection. Deep AVMs place the cerebellar nuclei at risk. The deep white matter feeders must be carefully handled to avoid their retraction into the deep cerebellum, leading to postoperative life-threatening posterior fossa expanding hematomas.
Figure 12: A relatively diffuse superior vermin AVM is depicted on lateral and AP vertebral angiograms (top row). This ruptured AVM underwent resection via a midline suboccipital approach (second row). The supracerebellar route exposed an AVM-draining superior vermin vein; note the arterialized vein joining the vein of Galen (arrow)(third row). The surface of the AVM in the culmen and its nidus are evident on the images of the bottom row.
Postoperative Considerations
Strict control of systolic blood pressure (~20% below the normal preoperative levels) is particularly important during postoperative care (2-3 days after surgery) of posterior fossa AVMs. Expanding clots related to hypertensive hematomas in the operative bed lead to irreversible and disappointing results.
Intraoperative angiography plays an especially important role because minor remnants of the AVM nidus can also lead to expanding hematomas.
These above two factors are especially applicable to cerebellar AVMs.
Contributor: Farhan A. Mirza, MBBS
References
Lawton MT. Seven AVMs: Tenets and Techniques for Resection. New York, Stuttgart: Thieme Medical Publishers, 2014.
Spetzler RF. Comprehensive Management of Arteriovenous Malformations of the Brain and Spine. Cambridge: Cambridge University Press, 2015
Please login to post a comment.