Pineal Region Tumor (Infratentorial Supracerebellar Approach)
This is a preview. Check to see if you have access to the full video. Check access
Resection of a Pineal Region Tumor via the Paramedian Supracerebellar Approach
Surgical approaches to pineal tumors tested the resolve of our early forefathers and were not reported until the early 1900s. Early pioneers of pineal surgery included Sir Victor Horsley, Brunner, and Schloffer, all with minimal or no success until Oppenheim and Krause in 1913. In 1921, Walter Dandy described the transcallosal approach in three patients with pineal tumors after he had developed the technique safely in puppies.
In 1926, Krause published a report of a successful infratentorial approach to pineal region tumors in three patients without operative mortality. These successes sparked interest in developing more effective approaches to the pineal region. But with minimal success, more conservative management prevailed until the advent of the operating room microscope.
In 1971, Stein explored Krause’s infratentorial supracerebellar approach and achieved resection of six pineal masses with minimal morbidity and no operative mortality. This success encouraged surgeons to reconsider pursuit of other surgical approaches to pineal tumors, including supratentorial ones.
Figure 1: Just about 100 years ago, Walter Dandy described the technique of using the posterior transcallosal route for safely removing the pineal gland in puppies.
Diagnosis
Patients with pineal region tumors most commonly present with symptoms of elevated intracranial pressure secondary to obstructive hydrocephalus, including headaches, visual and cognitive disturbances, and impaired gait. Symptoms of acute hydrocephalus, such as obtundation and loss of consciousness, may be evident and require emergent cerebrospinal fluid (CSF) diversion.
Alternatively, patients may present with signs of brainstem or cerebellar compression, including Parinaud’s dorsal midbrain syndrome of upgaze palsy, eyelid retraction, convergence nystagmus, and light-near dissociation. Patients may rarely demonstrate endocrine dysfunction due to hydrocephalus affecting the hypothalamus. In particular, diabetes insipidus may occur if a germinoma invades the floor of the third ventricle.
Evaluation
If a patient presents with acute mental status changes, a computed tomography (CT) scan evaluates the ventricular size, which, if significantly enlarged, necessitates CSF diversion via an external ventricular drain. For more information regarding diagnosis and evaluation of pineal region tumors, please refer to the chapter on Selection of Operative Corridor.
Other complementary imaging studies include magnetic resonance (MR) imaging with and without gadolinium enhancement to assess the tumor’s enhancement pattern, extent of mass effect, and the location of the deep venous drainage systems, all important considerations for surgical planning.
Most pineal region tumors displace the venous system, including the vein of Galen and its tributaries rostrally and dorsally along the periphery of the tumor capsule. Meningiomas from the velum interpositum, splenial epidermoids, and tectal/thalamic tumors tend to displace the venous structures caudally and ventrally: recognition of these anatomic variations is important and affects surgical planning.
In addition, the tumor’s size, vascularity, and its relationship to the surrounding structures, particularly the third ventricle, along with its extent of lateral/supratentorial extension and brainstem involvement, all influence the surgical approach and methodology. Although tumor margins may be estimated based on preoperative imaging, the true extent of invasiveness can only be determined intraoperatively.
If the regional vascular anatomy is not clear, CT or MR angiography or in rare instances catheter angiography can be helpful in elucidating the relevant pathoanatomy. The utility of preoperative embolization can also be evaluated. Velum interpositum and many of the pineal region meningiomas typically cannot be embolized because their dominant blood supply arises from the medial posterior choroidal arteries that are not accessible through endovascular means.
Laboratory studies, including CSF analysis for beta-human chorionic gonadotropin (beta-HCG), placental alkaline phosphatase (PLAP), and alpha-fetoprotein (AFP), can help distinguish germ cell tumors. Endodermal sinus tumors and immature teratomas are suspected when AFP is elevated. Elevation of beta-HCG suggests choriocarcinoma or, less commonly, embryonal cell carcinomas and germinomas. Of note, a lack of AFP or beta-HCG elevation does not necessarily rule out a germinoma or embryonal cell carcinoma; intraoperative frozen section results should be available before resection is conducted. Also, there may be mixed tumors expressing both markers (Table 1). PLAP may be used for immunohistochemical analysis, but is not as equally useful as a CSF marker.
| Tumor Type | Alpha-Fetoprotein | Beta-Human Chorionic Gonadotropin |
| Benign germ cell | - | - |
| Immature teratoma | +/- | ? |
| Germinoma | - | +/- |
| Embryonal cell carcinoma | - | +/- |
| Choriocarcinoma | - | ++ |
| Endodermal sinus tumor | ++ | - |
| Meningiomas | - | - |
CSF cytology is rarely diagnostic, but the presence of malignant cells can guide the necessary treatment paradigm without the need for further biopsy and tissue sampling.
The Underlying Pathology at the Pineal Region
The pineal region gives rise to many different types of benign and malignant tumors. The four main categories are germ cell tumors, pineal parenchymal cell tumors, glial cell tumors, and other tumors such as meningiomas, hemangioblastomas, choroid plexus papillomas, chemodectomas, lymphomas, and metastatic tumors.
Vascular malformations are less common, but do occur. These include arteriovenous malformations, cavernous malformations, and vein of Galen malformations.
Benign pineal cysts are the most common mass within the pineal region. Radiographically, these lesions demonstrate a contrast-enhancing rim and may be as large as 2 cm. They are benign, normal variants of the pineal gland with cystic structures surrounded by normal pineal parenchymal cells. They are typically asymptomatic, do not progress and do not require treatment unless they cause aqueductal obstruction. I believe these lesions can only rarely cause nonspecific symptoms such as headaches in the absence of hydrocephalus or other secondary structural abnormality.
Meningiomas of the posterior third ventricle and pineal region are rare. These tumors are thought to arise from arachnoid cap cells within the velum interpositum at the roof of the third ventricle and pineal region. Although meningiomas can originate from anywhere within the third ventricle, they are commonly divided into anterior and posterior third ventricular meningiomas.
Figure 2: Various pathologies within the pineal region are illustrated. The upper row demonstrates a pineal cyst. Note the thin rim of enhancement without any other unusual enhancing features. The lower row demonstrates a pilocytic astrocytoma of the tectum. For other pathologies, including a germinoma, pineoblastoma and hemorrhagic glioma, refer to the chapter on Selection of Operative Corridor.
This chapter refers to tumors of the pineal region and of the posterior third ventricle that extend into the pineal region.
Indications for Surgery
Treatment of pineal region tumors typically requires an initial plan for CSF diversion because obstructive hydrocephalus is a common cause of presenting symptoms.
Definitive treatment of nongerminomatous tumors is surgical resection, and gross total removal is the goal. The diagnosis is not always known preoperatively based on imaging and laboratory tests. Tissue sampling may be necessary to ensure the need for gross total resection and, more importantly, to avert its risk if it is unnecessary. For further information regarding the decision making process for management of pineal region tumors, please refer to the chapter on Selection of Operative Corridor.
There is no uniformly accepted protocol for the evaluation and management of pineal region tumors. Some surgeons prefer an initial stereotactic biopsy followed by gross total resection if an operative pineal region tumor is confirmed based on pathological examination. This process prevents an unnecessary open surgery, but in cases where gross total resection is indicated, it subjects the patient to two operations.
Also, needle biopsy of pineal region tumors carries a greater risk of hemorrhage than in other parts of the brain because of the adjacent vascular structures, crossing of more than one pial plane, lack of surrounding tissue to provide tamponade, and high hemorrhage rates of the pathologies encountered within the pineal region.
Some colleagues prefer to plan for a gross total resection of any symptomatic tumor, and if the pathology revealed by intraoperative frozen sections does not dictate the need for gross total resection, a less aggressive resection strategy is pursued. Moreover, an open biopsy allows for more thorough tissue sampling, which may be particularly important because of the possibility of heterogeneity in germ cell tumors.
Management of symptomatic hydrocephalus favors an endoscopic third ventriculostomy (ETV) over ventriculoperitoneal shunting, especially among younger patients with long life expectancies. ETV avoids the risk of peritoneal seeding of malignant cells. Mild, asymptomatic hydrocephalus may not require treatment because tumor resection facilitates its resolution.
Endoscopic biopsy may be paired with endoscopic third ventriculostomy in order to treat hydrocephalus and obtain tissue diagnosis during a single operation. My preferred decision making process for treatment of pineal region tumors is summarized in the following figure.
Figure 3: The recommended decision tree to deal with uncertainties associated with the diagnosis of a pineal region tumor is indicated.
Benign masses and nongerminomatous tumors respond effectively following their gross total resection. Densely adherent tumors necessitate subtotal resection to preserve deep venous structures and brainstem pial planes.
Preoperative Considerations
Preoperative hydrocephalus is addressed through an external ventricular drain or third ventriculostomy.
For execution of the posterior fossa surgery, morbidly obese patients with short necks may benefit from the sitting position. This position will facilitate ventilation and cranial venous return; both of these parameters can be compromised in the lateral and prone positions in morbidly obese patients. Appropriate preoperative preparations should be made to avoid and manage venous air embolism. I do not routinely use neurophysiologic monitoring for pineal region surgery.
Figure 4: A careful assessment of the deep venous system in select patients is beneficial during preoperative evaluation. This immature teratoma was evaluated via a CT angiogram that demonstrated displacement of the vein of Galen and its tributaries posteriorly (blue arrow). This venous configuration prohibited an effective exposure of the tumor via the supracerebellar approach and the occipital transtentorial route was deemed more appropriate.
Operative Anatomy
The pineal gland is located in the center of the brain. Fortunately, a relatively clear surgical plane can often be established between the adjacent structures to reach pineal tumors.
The adjacent structures are the posterior commissure, the corpus callosum, and the habenular commissure. The dorsal internal cerebral veins join the basal veins of Rosenthal to form the vein of Galen before draining into the straight sinus. The blood supply of these tumors is from branches of the medial and lateral posterior choroidal arteries with multiple anastomoses to the pericallosal, posterior cerebral, superior cerebellar, and quadrigeminal arteries.
There are several approaches to the pineal region; the main routes of interest are divided into supratentorial and infratentorial routes. These approaches are discussed in the Pineal Region Tumors: Selection of Operative Corridor chapter.
The infratentorial routes are divided into midline and paramedian supracerebellar approaches. The supratentorial approaches include the occipital transtentorial, interhemispheric transcallosal and transcortical transventricular approaches.
The supratentorial approaches are reserved for large tumors extending supratentorially or laterally into the trigone or if the ventrally displaced deep veins prohibit the infratentorial routes. I prefer the infratentorial approaches in almost all cases unless the tumor harbors a dominating supratentorial component or if the displaced deep veins are obstructive. The infratentorial approaches are associated with less risk of neurologic morbidity.
Figure 5: The typical approaches to the pineal region are summarized. The left image demonstrates the operative trajectory for supracerebellar infratentorial (a) and occipital transtentorial (b) trajectories. The right image demonstrates the most commonly used midline supracerebellar infratentorial approach (a); the paramedian variants are more favorable in my opinion (b). The occipital transtentorial route is marked with c (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
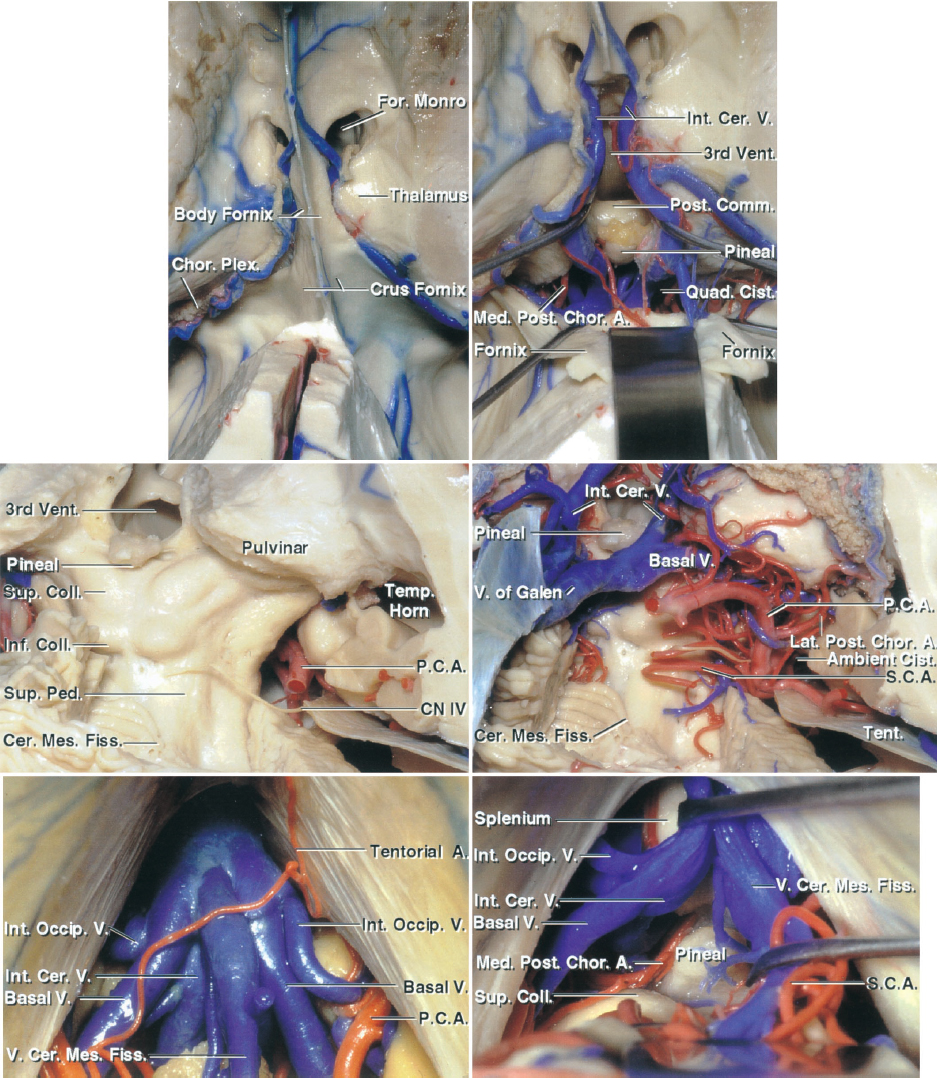
Figure 6: The above images compare the midline infratentorial supracerebellar and the occipital transtentorial approaches to the pineal region and the posterior third ventricle. The first row demonstrates the superior view to the intraventricular anatomy in relation to the pineal region. Note the location of the forniceal bodies and the venous anatomy (the fornices have been reflected posteriorly in the right upper image). The second row demonstrates the vascular anatomy and the location of the trochlear nerve via the occipital transtentorial approach. The bottom row indicates the midline infratentorial supracerebellar approach. The venous anatomy includes the internal occipital, basal and internal cerebral veins, and the vein of the cerebellomesencephalic fissure (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 7: The anatomic relationships of the structures surrounding the pineal region are reviewed. The paramedian supracerebellar approach reaches over the lower slope of the lateral supracerebellum compared with the midline supracerebellar trajectory that works around the apex of the cerebellum (culmen). Cranial nerve (CN) IV is typically the inferior margin of the resection cavity. The indispensable arterial and venous structures are demonstrated in the images of the lower row (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 8: The operative view through the midline infratentorial supracerebellar approach and the relevant venous complex joining the vein of Galen are indicated. This complex contains the internal occipital, basal, and internal cerebral veins and the vein of the cerebellomesencephalic fissure (transected). The vein of Galen has been moved to expose the splenium (left lower image). The pulvinar forms the lateral margins of the operative field. The lesion usually but not always displaces the deep veins anteriorly and dorsally (images courtesy of AL Rhoton, Jr.).
Click here to view the interactive module and related content for this image.
Figure 9: Pineal region as seen from a left-sided paramedian supracerebellar infratentorial approach (images courtesy of AL Rhoton, Jr). This approach is more effective for reaching the midline pineal region and is discussed in the Paramedian Supracerebellar Craniotomy chapter.
INFRATENTORIAL SUPRACEREBELLAR RESECTION OF PINEAL REGION TUMORS
The deep location of the pineal region and its associated vascular structures create a surgical challenge. For a discussion of the available surgical approaches to this region, please see the chapter titled Selection of Operative Corridor.
Craniotomy
For more details regarding the nuances of technique for the initial stages of exposure, see the midline supracerebellar craniotomy chapter. Please note that I routinely use the paramedian supracerebellar craniotomy for pineal region masses, regardless of their size. The midline counterpart is discussed here because most colleagues are familiar with this approach that is most commonly practiced.
INTRADURAL PROCEDURE FOR MIDLINE SUPRACEREBELLAR CRANIOTOMY
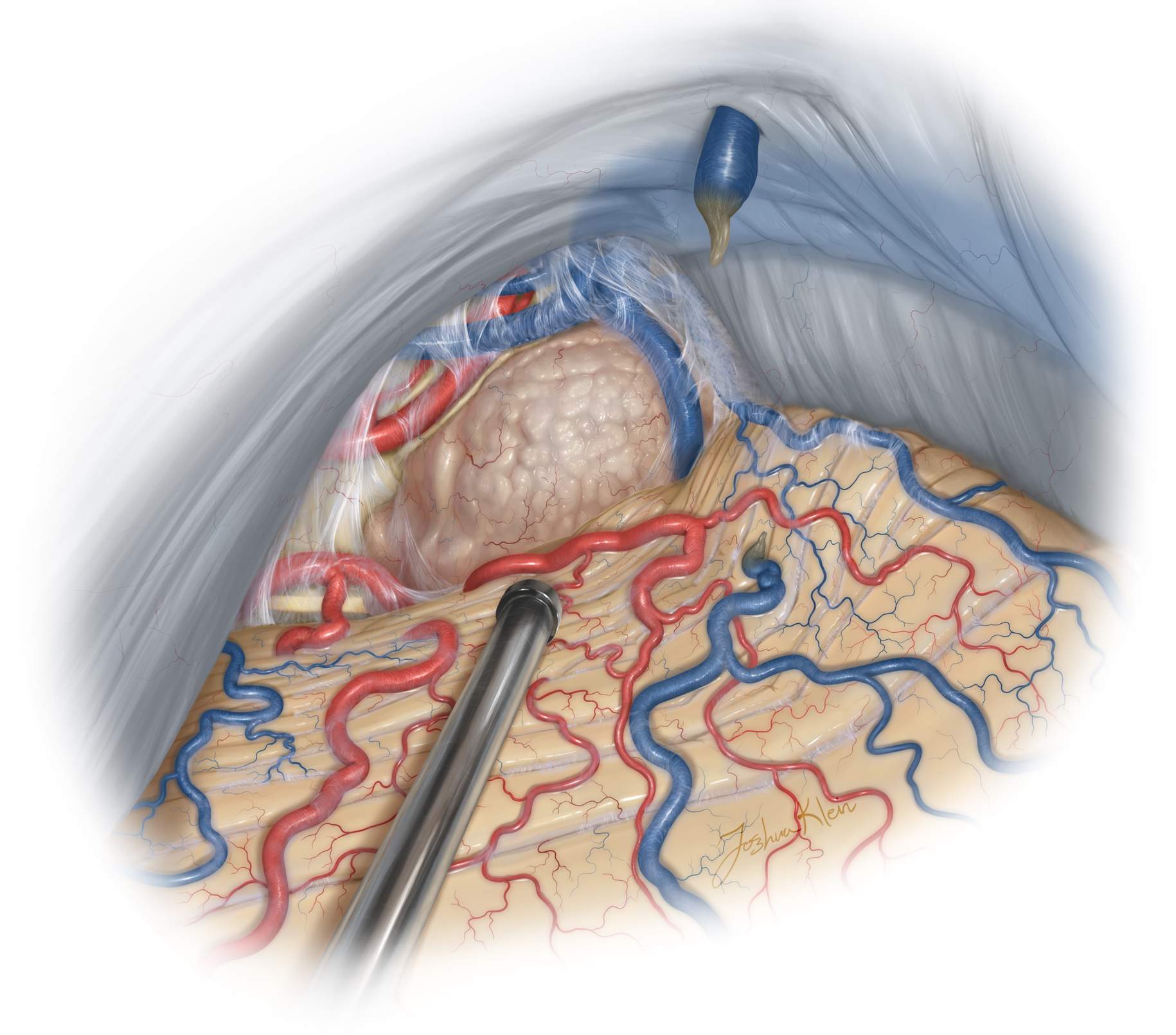
During inferior mobilization of the midline cerebellum, one or two midline bridging veins are sacrificed. This maneuver can lead to cerebellar venous congestion, limiting the exposure, and therefore should be minimized.
Figure 10: The precentral cerebellar vein leading to the superior vermian vein can be sacrificed safely (top sketch). This vein travels from the anterior vermis toward the vein of Galen. Other more anterior diencephalic veins residing on the brainstem should be preserved. There is a thick veil of arachnoid encasing the precentral vein (lower intraoperative photo, left). The cerebellum gradually descends with dissection of the supracerebellar arachnoid bands, exposing the pineal region and the tumor (lower intraoperative photo, right).
Figure 11: During supracerebellar dissection and as I follow the contour of the midline tentorium toward the pineal region, I find myself heading toward the vein of Galen and splenium. I redirect my operative trajectory inferiorly or ventrally at the deep portion of the operative field to avoid blind dissection around the diencephalic venous confluences.
Figure 12: The operative trajectory is further directed inferiorly. Dynamic retraction of the handheld suction device mobilizes the apex of the cerebellum (culmen) and exposes the exact area of the tumor, while minimizing fixed retraction injury on the surrounding tissues. The arachnoid membranes over the posterior capsule of the tumor are released. The deep veins are often not in view yet because they are displaced dorsally and laterally. If preoperative assessment of the deep veins was not conducted, the vein of Galen may be occasionally found posterior to or within the tumor capsule. The surgeon should remain on the look out for the veins.
Figure 13: The rest of the dissection follows the basic principles of tumor microsurgery, namely, tumor debulking and devascularization followed by extracapsular dissection. The tumor capsule is coagulated and generous pieces of specimen are sent for pathologic analysis. The heterogeneity of the lesions occurring in this area, combined with the unavailability of reliable preoperative diagnostic tools, demands accurate analysis of the initial tissue by our pathology colleagues before gross total resection is attempted. It is important to wait for the frozen section analysis before attempting aggressive resection.
Figure 14: Following adequate tumor decompression, the capsule is dissected, first laterally from the tectum, and then inferiorly from the anterior culmen, and ultimately superiorly from the vein of Galen and its tributaries. Pial planes around the pulvinar are preserved when feasible. If the tumor encases the veins, a conservative approach is appropriate and a small sheet of tumor is left behind to avoid their minor injury. After removal of the tumor, the inlet into the third ventricle is apparent (Redrawn from Tew, van Loveren, Keller*).
If pial violation around the tectum is unavoidable during resection of an otherwise benign resectable tumor, I proceed with gross total tumor removal at the expense of minor injury to the tectum. This maneuver leads to temporary Perinaud’s syndrome that resolves within a month after surgery.
Figure 15: After removal of the lesion, the compressed normal anatomy is evident. The CN IV and its encasing arachnoid is adherent to the inferior tumor capsule; this arachnoid, and not the nerve, can be carefully handled to mobilize the nerve (Redrawn from Tew, van Loveren, Keller*).
My Preferred Route for Resection of Pineal Tumors: The Unilateral Paramedian Supracerebellar Route
I prefer the left-sided paramedian supracerebellar infratentorial approach for most tumors in the pineal region. This approach provides a generous corridor by using the lateral path over the inferiorly-sloped supracerebellar surface, which can reach the more inferiorly located tumors than the midline corridor over the culmen.
There are fewer bridging veins lateral to the midline that need to be interrupted for access to the pineal region. The lower slope of the lateral supracerebellum decreases the need for fixed and aggressive retraction on the cerebellum. Visualization of the contralateral extent of the tumor is more than adequate based on the operative cross-court trajectory. Gross total resection of large pineal region tumors is readily possible. Extent of resection is limited by fibrous tumors that are adherent to the surrounding neurovascular structures.
Figure 16: The operative trajectory of the left paramedian infratentorial supracerebellar approach to the pineal region is illustrated. The tentorium may be sectioned to advance the supratentorial reach. The oblique trajectory of this approach can be disorienting to the surgeon.
Figure 17: The surgeon's operative view via the paramedian infratentorial supracerebellar approach to the pineal region is shown. The midline draining veins are avoided and the lateral wing of the cerebellum provides a more unobstructed exposure of the pineal region. The CN IV is at the most inferolateral corner of the field.
Figure 18: This non-traditional lateral trajectory can be disorienting to the surgeon. The third ventricle in ghosted in blue anterior to the tumor to illustrate the risk of penetrating the contralateral wall of the ventricle if the operator is not aware of the underlying anatomy deep to the tumor based on the working angle of the dissection.
Figure 19: The following three images further demonstrate the cross-court operative trajectory toward the pineal region and the third ventricle via the paramedian infratentorial supracerebellar approach. The middle image includes the location of the third ventricle in blue. The surgeon should appreciate the oblique anatomical position of the ventricle and not violate the wall of the contralateral third ventricle along the depth of dissection. The normal anatomy is simplified in the last image.
Figure 20: A left-sided paramedian supracerebellar route was used to resect a pineoblastoma. Note CN IV on the left side of the top photo. The branches of the superior cerebellar artery were mobilized. The bottom image demonstrates the posterior tumor capsule via the unilateral approach.
Figure 21: The vein of Galen was found engulfed within the tumor. A small portion of the tumor was left behind around the vein (top). The walls of the third ventricle were identified after removal of the mass. Note the orientation of the ventricle via the unilateral supracerebellar corridor.
Other Considerations
The caution for preservation of the deep veins cannot be overemphasized. The vein of Galen can be displaced caudally or posteriorly and therefore be at high risk of injury during the supracerebellar exposure of the tumor.
Figure 22: This hemorrhagic low-grade astrocytoma of the pulvinar (left upper image) had displaced the vein of Galen posteriorly (right upper image-blue arrow). Despite my attempts to stay vigilant about identifying the vein within the mass, I almost injured the vein during the surgery as it was embedded within the tumor. Postoperative scans (lower images) reveal the planned subtotal resection of the tumor. The intrinsic thalamic components of the tumor were left intact. This case re-emphasizes the importance of careful analysis of the preoperative imaging studies.
Hemorrhagic Pineal Region Tumor: Anatomic Challenges
Closure
Once the tumor is removed from the field, absolute hemostasis is achieved. Any clot within the third ventricle is evacuated. The dural flap is then released and closed primarily, if possible. If necessary, a dural substitute is sewn in place or laid over the defect. Watertight dural closure is advised.
Postoperative Considerations
The patient is monitored in the Intensive Care Unit for 24 to 48 hours postoperatively. Careful and frequent neurologic examinations are necessary; temporary lethargy is not uncommon after procedures involving brainstem manipulation.
Some patients experience impairment in extraocular movements, particularly upgaze and convergence, although this is typically transient and resolves within the first few days to weeks. These complications are more common in patients with progressive preoperative symptomatology, previous radiation, and invasive tumors.
Steroids are typically continued for the first few days and gradually tapered. If the patient has a ventriculostomy and no permanent CSF diversion, drainage is continued for 48 to 72 hours postoperatively. If obstructive hydrocephalus persists upon clamping the drain, the patient is most likely a candidate for permanent CSF diversion, preferably an endoscopic third ventriculostomy.
Posterior fossa surgery can result in chemical meningitis. Intractable worsening headaches, severe nausea/vomiting, dizziness, vertigo, and neck stiffness are all possible symptoms. Chemical meningitis is usually self-limiting and may be treated with steroids if necessary.
Patients should have an enhanced postoperative MRI within 72 hours of their surgery to evaluate the extent of resection. For patients with confirmed malignant germ cell tumors or ependymomas, a spinal MRI should be performed to check for spinal metastasis. Some surgeons choose to delay spinal imaging because of the potential of postoperative debris and blood clots mimicking spinal metastasis.
Radiation therapy is required for patients with malignant germ cell or pineal cell tumors at a dose of 5500 cGy, given in 180-cGy daily fractions, 4000 cGy to the ventricular system, and an additional 1500 cGy to the tumor bed. Germinomas are the most radiosensitive malignant tumors, with tumor control in up to 90% of cases. Radiation therapy is unnecessary if gross total resection has been achieved for a histologically benign pineocytoma or ependymoma. Currently, prophylactic spinal irradiation is not recommended for malignant pineal tumors. If spinal MRI reveals seeding, a dose of 3500 cGy is recommended.
Chemotherapy has been shown to be beneficial for malignant nongerminomatous germ cell tumors. Commonly, testicular cancer regimens such as the Einhorn regimen of cisplatin, vinblastine, and bleomycin have been used, in addition to cyclophosphamide or etoposide. In an attempt to reduce lung toxicity from vinblastine and bleomycin, etoposide has been used with cisplatin or carboplatin and has shown improved response rates.
Pearls and Pitfalls
- Pineal region tumors should be carefully studied preoperatively to ensure the need for surgical resection. Tumors amenable to nonoperative therapy should be excluded from surgical consideration.
- The paramedian infratentorial supracerebellar approach places only one of the transverse sinuses at risk, spares the torcula from operative manipulation and minimizes the risk to supracerebellar bridging veins.
*Redrawn with permission from Tew JM, van Loveren HR, Keller JT. Atlas of Operative Microneurosurgery, WB Saunders, 2001. © Mayfield Clinic
References
Al-Mefty O. Operative Atlas of Meningiomas. Lippincott-Raven, Philadelphia, PA; 1998.
Bruce JN. Pineal tumors, in Winn HR, Berger MS, Dacey RG (eds): Youmans Neurological Surgery, 6th ed. Saunders, Philadelphia, PA; 2011:1359.
Dandy WE. An operation for the removal of pineal tumors. Surg Gynecol Obstet 1921; 33:113.
Ellis J, Moise G, Bruce J. Meningiomas of the third ventricle and pineal region, in Demonte F, McDermott M, Al-Mefty O (eds): Al-Mefty’s Meningiomas, 2nd ed. Thieme, New York, NY; 2011:323
Krause F. Operative frielegung der vierhugel, nebst beobachtungen uber hirndruck und dekompression. Zentrabl Chir 1926;53:2812.
Oppenheim H, Krause F. Operative erfolge bei geschwulsten der sehhugelund vierbugelgegend. Berl Klin Wochenshr 1913; 50:2316
Parker J, Waziri A. Preoperative evaluation of pineal tumors. Neurosurg Clin N Am 2011; 22:353–358.
Rhoton AL Jr. The tentorial incisura. Neurosurgery 2000;47(Suppl 3):93–129.
Shinoda J, Yamada H, Sakai N, et al. Placental alkaline phosphatase as a tumor marker for primary intracranial germinoma. J Neurosurg 1988;68:710–720.
Stein BM. The infratentorial supracerebellar approach to pineal lesions. J Neurosurg 1971;35:197–202.
Please login to post a comment.