Skull Base Reconstruction and CSF Leak Repair
Any endoscopic endonasal skull base procedure may be divided into the same three phases described for traditional “open” skull base operations: 1) approach (exposure), 2) definitive procedure (resection), and 3) reconstruction (closure). Our rhinology colleagues primarily undertake the first and last phases of the operation and drive the endoscope during the resection phase. Importantly, this team approach and mutual learning advances the care of our patients.
Each of these phases requires a different set of goals and objectives as well as techniques. In this chapter, I will discuss the basic tenets of surgical technique for skull base reconstruction and cerebrospinal fluid (CSF) leak repair.
Once hemostasis is obtained, a decision point has been reached. In an extradural procedure where no cerebrospinal fluid leak occurs (such as an intrasellar tumor with an intact sellar diaphragm or a clival bony tumor without dural invasion), closure is relatively straightforward. A small piece of fat globule harvested from the abdomen (bare or wrapped in an absorbable hemostatic agent) may be placed over the skull base defect and held in place with fibrin glue. This barrier will protect the dura from the contents of the nasal sinuses.
REPAIR OF CEREBROSPINAL FLUID LEAKS
These fistulas are divided into simple (low flow) and complex (high flow) categories according to the techniques required for their repair.
Simple (Low Flow) Cerebrospinal Fluid Fistulas
If CSF flow is seen, a second decision point occurs. A small, low-flow leak such as the one that occurs through a small tear in the sellar diaphragm can be plugged with a piece of fat globule. I wrap the fat globule in a piece of Surgicel; this maneuver allows me to handle or manipulate the fat globule (“surgifat”) precisely toward the point of the leak without its disintegration.
I mobilize the surgifat within the sella and make sure the fat plugs the exact site of the leak. I avoid indiscriminate and nontargeted packing of the entire sella because this technique would not only ineffectively seal the defect, but it can cause optic apparatus compression.
More specifically, the exact site of the leak is identified meticulously and the surgifat is placed over the defect. Next, additional pieces of surgifat are used to buttress the initial piece of surgifat and hold it in place. Often the leak is located as the point of attachment of the arachnoid to the tuberculum, and all that is needed to seal the defect is to hold the arachnoid (with the pituitary gland attached to it) in place against the tuberculum by buttressing the pituitary gland with a piece of surgifat.
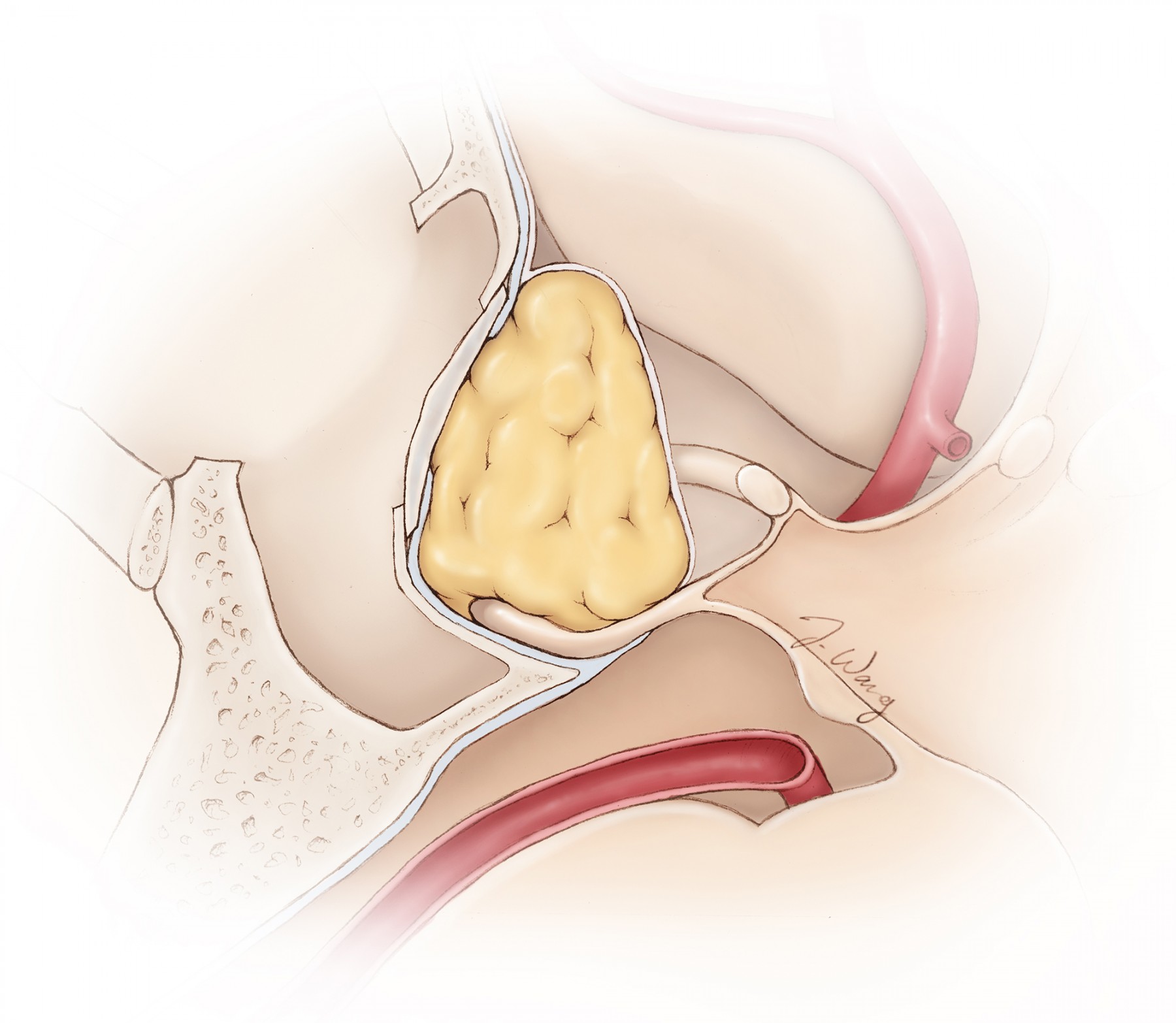
Figure 1: A tear within the diaphragm and resultant CSF leakage often occurs during blind forceful removal of a tumor located along the anterior aspect of the resection cavity above the tuberculum sella (anterior sellar recess) where the diaphragm is attached (inset image). It is important to identify the diaphragm and maintain its integrity throughout resection. If an inadvertent leak is apparent, I pack the previously prepared fat globules from the patient’s abdomen at the exact location of the leak and avoid indiscriminately and forcefully packing the entire sella because this maneuver could lead to suprasellar mass effect and vision impairment.
Figure 2: The fat globules are wrapped in pieces of Surgicel to create spheres. This method facilitates their handling and directing their insertion/placement to the exact site of the leak. The reconstruction is targeted to the site of the leak. A piece of bony septum or prosthesis is used to keep the fat in place and reconstruct the sella floor. If Valsalva maneuvers exclude a leak, I still gently pack the sella to seal a potentially occult leak.
Complex or High-Flow Cerebrospinal Fluid Fistulas
Larger, high-flow leaks where the cisternal contents (cerebrovascular structures) are visible or the ventricle (third chamber) is entered carry a high risk of postoperative CSF fistula. Hence, the closure is layered using the nasoseptal flap.
First, an inlay of dural substitute is tucked intradurally: all edges of the graft should remain occlusive. This closure is checked under normal ventilation to confirm the absence of any obvious leakage. Next, the nasoseptal flap is used to cover the bony defect and surrounding bone edges. Larger transcribriform defects may also benefit from an additional layer of inlay/overlay. The edges of the overlay dural substitute are inserted into the epidural space. Next, the nasoseptal flap is used to cover the defect.
If the bony defect is relatively medium to small in size and its surface does not contain highly acute angles, an alternative and more reliable relatively rigid closure technique is available and is my preference. An overlay of dural substitute is placed over the bony edges. The radius of the graft must exceed the bone defect by at least 1 cm. A solid buttress is then placed using the “gasket-seal technique” to provide form-fitting support for the dural repair. A piece of rigid material such as Porex (Porex Corp, Newnan, GA) is fashioned to be roughly the size of the bone defect.
The rigid plate is gently countersunk into the bone defect; subsequently, the tissue allograft is drawn into the defect, forming a watertight gasket seal around the rigid prosthesis. The construct is then inspected for leakage using a Valsalva maneuver. A residual leak will require the construct to be redone. If the optic nerves were unroofed, the construct should not compromise the nerves via compression; notches are made in the Porex where the Porex comes in contact with the nerves.
I believe that the solid construct, when feasible, exponentially decreases the risk of CSF leakage compared with other soft constructs that do not employ the gasket-seal technique.
Fibrin glue may be used to further reinforce the construct, but its effectiveness is controversial. Finally, a vascularized mucosal flap, usually a nasoseptal flap, is placed over the defect, while the surgeon takes care to ensure that the flap edges are in direct contact with bare bone circumferentially around the defect. I strip the bony edges of the defect of any secretory mucosa to allow flap adherence to the bone, minimizing the risk of flap dehiscence. A single layer of Surgicel is placed around the edges of the flap to hold it in position and prevent flap migration.
The important aspects of reconstruction using the flap include target-specific flap design and harvest, pedicle protection, preparation of bone defect and graft site, multilayer closure, maximization of the flap reach, final positioning, and proper buttressing of the flap. Our rhinologist colleagues play a vital role in the closure stage.
Nasal gloved Merocel Sinus Packs (Medtronic, Minneapolis, MN) are placed to gently hold the flap against the skull base. Finally, intrathecal fluorescein injection by means of the lumbar drain can be helpful to localize the small leakage sites that might go unnoticed.
Figure 3: Once tumor resection is complete and the involved dura has been resected and/or cauterized, a gasket closure technique is used by first covering the bone defect with an oversized dural substitute or fascial graft, followed by a countersunk rigid implant: Porex (Stryker, Kalamazoo, MI). I do not intradurally pack the suprasellar space or tumor cavity with fat or foreign materials in order to avoid unintended optic nerve compression and facilitate interpretation of postoperative images. Valsalva maneuvers are used to inspect for any obvious CSF leakage before the next step in reconstruction is attempted. The nasoseptal flap is then placed over the initial closure so that the flap is in direct apposition to the surrounding bony skull base and subsequently held in place with DuraSeal (Covidien, Dublin, Ireland) or fibrin glue. Bilateral nasoseptal flaps may need to be used for larger skull base defects after transcribriform osteotomies. These larger defects are not amenable to the gasket-seal technique.
A lumbar drain is inserted at the beginning of the operation. I open the lumbar drain on the first postoperative day and remove 5 to 10cc of CSF per hour. The lumbar drain is discontinued about 2 to 4 days after surgery to facilitate early mobilization.
Giant Defects and Special Circumstances
The septal mucosal flap may be unavailable for reconstruction. This can be because of prior procedures, failure of the nasoseptal flap, or involvement of the nasal septum by sinonasal cancers.
In these situations, a piece of frontal pericranial flap (please see the extracranial pericranial flap section below) can be harvested for skull base reconstruction. This flap can be obtained using open (via a bicoronal incision) or endoscopic (via two small stab incisions just behind the hairline) techniques.
RECONSTRUCTION TECHNIQUES
Preoperative planning for reconstruction is a pivotal requirement for successful skull base repair that is reliant upon thorough computed tomographic (CT) analysis in the axial, sagittal, and coronal planes. Magnetic resonance (MR) imaging may also be needed for dural defect evaluation by defining the extent of the meningeal defect and the condition of adjacent cerebral structures.
Additional preoperative considerations for the repair of a dural defect include: the use of lumbar drainage to decrease intracranial pressure and fluorescein dye localization of the difficult-to-detect dural defects. Lumbar drainage can also serve as a method of supplementing dural defect closure during the postoperative period.
Dural defects smaller than 1-2 cm can be closed using nonvascularized autologous or heterologous grafts. Larger defects, greater than 2 cm, should be reconstructed using vascularized flaps, among which the nasoseptal flap is the preferred method. Alternatives that will also be discussed in this chapter include middle and inferior turbinate flaps, extracranial pericranial flaps, and temporalis muscle flaps. Vascularized grafts must be considered in settings when there are no bone edges, regardless of the defect size, due to the difficulty of correctly placing the inlay grafts.
Nonvascularized Repair of Small Dural Defects
The technique of nonvascularized repair of a dural defect typically involves the use of a piece of autologous (fascia lata or adipose tissue graft) or less commonly heterologous graft. Acquisition of the autologous graft should be preoperatively planned and considered during patient positioning. The patient is positioned in the lateral decubitus position upon entering the operating room to facilitate lumbar puncture, lumbar drain insertion, and/or injection of diluted fluorescein (0.25mL of 10% fluorescein).
Following the lumbar puncture and general anesthesia, the patient is placed in the supine position. A Mayfield skull clamp is applied and image guidance is configured to assist in intraoperative localization of the dural defect. A vasoconstrictive agent is applied to a cottonoid or neuropledget, which can be inserted into the nares, permitting application and subsequent decongestion of the nasal mucosa.
Next, the surgeon should attempt direct visualization of the skull base defect, utilizing an angled rigid endoscope (30- or 45-degree). Intrathecal fluorescein may be employed to reveal small and difficult-to-reach fistulas.
After visualizing the defect margins along the skull base, these bone margins should be cautiously separated from the overlying mucosa. Gentle manipulation of the defect margins is paramount due to the potential risk of enlarging the defect with more aggressive instruments.
The choice of autologous homograft is largely dependent on the characteristics of the dural defect. A dural defect under low pressure and with a low volume output is amenable to an underlay adipose tissue graft. Following the successful placement of the underlay graft, a blue light endoscope can be used to observe for a watertight closure. Additional closure augmentation can be achieved with the use of fibrin glue or other commercially available related products.
Slightly larger defects (2 x 2.5 cm) and those with high-volume flow are best repaired via a gasket-seal closure method using an autologous fascia lata graft or a piece of allograft dura. The graft size should always be larger than the defect, but not so large that it cannot be manipulated as an insert into the defect. Please see the above sections for further details regarding the gasket-seal technique.
Nasoseptal Flap
This method of vascularized dural defect closure is the most common, versatile, and recommended type for reliable repair of leaks. The flap is pedicled off the sphenopalatine artery along the posterior septal branch. The most common sites of application for the nasoseptal flap are the dural defects along the anterior fossa, middle fossa, and clival regions of the skull base.
Potential deferring features that may prevent the surgeon from using the nasoseptal flap include: a large septal perforation, posterior septectomy, prior sphenoidotomy or transpterygoid surgery with ligation of the posterior septal artery, and any endovascular embolization of the internal maxillary artery branches. As mentioned previously, invasion of the septum by sinonasal cancers is another contraindication for the use of this type of flap.
Preoperatively the surgeon should review detailed CT and MRI imaging to calculate the appropriate sized flap and ensure appropriate septum tissue is available. The side from which the flap will be harvested must be determined before surgery. Generally, the right side is the preferred side for flap harvest.
Following general anesthesia, the patient is placed in a supine position. A vasoconstrictive agent is applied to a cottonoid or neuropledget, which can be inserted into the nares, permitting application and subsequent decongestion of the nasal mucosa. On the side of the anticipated flap harvest, the septum is infiltrated with a solution of 1% lidocaine with 1:100,000 epinephrine.
Next, the inferior turbinate and usually the middle turbinate are resected. Resection of the inferior two-thirds of the superior turbinate and posterior ethmoidectomy follows. These maneuvers facilitate the creation of the superior incision for a flap that has its origin at the ostium of the sphenoid sinus. The surgeon should then complete the inferior incision.
The inferior incision should begin at the lateral margin of the choanal roof, progressing more medially onto the vomer, and then anteroinferiorly until meeting the nasal floor. Lastly the incision should be carried to the squamociliary junction. Monopolar cautery is the ideal method of making this incision, both for its speed and hemostatic ability.
Following completion of the inferior incision, the ostium of the sphenoid sinus is identified and the superior incision is carried anterosuperiorly until reaching a point 1cm below the skull base. Then the incision is carried anteriorly within the same vertical plane until it reaches the same point along the incision plane that the inferior incision ended. At this point a vertical incision is made to connect the inferior and superior incisions.
If a larger flap is anticipated, the inferior incision may be extended into the inferior meatus and the superior incision can be carried onto the cribiform plate. If a smaller flap is desired for the anticipated closure, the anterior margin of the septal incision may be made more posteriorly along the septum.
The flap should be freed along the mucosal surface to permit elevation along the submucoperichondrial and submucoperiosteal planes. The use of endoscopic scissors and an elevator is often necessary. Once the flap is elevated, it can be rotated laterally and applied to the skull base surface of interest. If additional surgical procedures are intended, that is, skull base tumor resection, the nasoseptal flap can be stored in the nasopharynx until the desired time of application. The surgeon must be conscious of the pedicle of the flap during the additional surgical procedures so as to avoid its vascular compromise.
When the flap is ready to be applied to the skull base surface, the pedicle should be examined to ensure an absence of rotation/kinking. The mucosal side of the flap should face externally. Excessive folds in the flap can lead to formation of late mucoceles.
Multilayered closure can be considered in combination with a nasoseptal flap through the use of a collagen matrix material as an underlay to attempt reformation of the arachnoid membrane. Surgicel and fibrin glue can then be applied as an overlay on the nasoseptal flap. A layer of gelfoam can then be implanted as the final layer.
Middle and Inferior Turbinate Flaps
These vascularized flaps are alternatives to the nasoseptal flap. These are generally small in size (~4cm2) compared with the nasoseptal flap (~25cm2). These flap closures are generally considered only when the nasoseptal flap is unavailable, as discussed in the section above.
The inferior turbinate flap is pedicled to include the inferior turbinate branch of the posterolateral nasal artery. The middle turbinate flap includes the middle turbinate branch of the sphenopalatine artery.
Both flaps have indications for the specific regions of the skull base amenable to their coverage. The inferior turbinate flap can be used to close the defects along the posterior cranial fossa or clivus. The middle turbinate flap can be used for closure of defects within the sella, fovea ethmoidalis, or planum sphenoidale.
Extracranial Pericranial Flap
The pericranial flap provides a vascularized tissue barrier separating the intracranium and nasopharynx. Settings where a pericranial flap is indicated include a large anterior skull base defect where the dura was resected and a CSF fistula persists despite the use of a nasoseptal flap. Previously radiated tumors that are undergoing repeat resections and unavailability of the nasoseptal flap are reasonable indications for the use of this form of flap.
The pericranial flap can be harvested via a transcranial (discussed first) or an endoscopic method.
Open Pericranial Flap Harvest Technique
The acquisition of the pericranial flap requires a wide bicoronal incision. The loose areolar layer deep to the galea is identified and permits dissection along the supraperiosteal plane. The extent of dissection depends on the size of the pericranial flap that is desired. If the supraperiosteal dissection is carried to the maximal extent in the anterior and posterior directions as permitted by the incision, a maximal pericranial graft of up to 10cm in length can be acquired. Once the desired pericranial flap dimensions are determined, electrocautery can be used to separate the flap from the temporalis muscle margins and carried in the desired length in the anterior and posterior directions.
The incision is extended within the superficial layer of the deep temporal fascia. There is an adipose layer between the superficial and deep layers of the deep temporal fascia that will be encountered. This incision and subsequent dissection can be carried down to the level of the zygomatic arch.
Once the zygomatic arch is reached, the dissection continues anteriorly until the lateral orbital margin is encountered. The flap is raised from the frontal bone and discontinued 10 mm rostral to the orbits. The remaining anterior margin of the pericranial flap bilaterally serves as the pedicle for rotation of the flap.
Rotation of the flap is largely dependent on the planned location of the flap repair. Repair of dural defects with a pericranial flap usually involves placement of a dural patch over the defect with an onlay of pericranial flap. This achieves a multilayered closure. The surgeon must be attentive to avoid compromising the axial blood supply to the flap, particularly when replacing the frontal bone flap.
Endoscopic Pericranial Flap Harvest Technique
This method is similar to the one described above except that this flap is pedicled unilaterally and involves a 5cm area within the coronal plane. This method avoids the full coronal incision for flap harvest, which is required for the open technique. Prior to making the incision, a Doppler probe is used to localize the supraorbital and supratrochlear arteries. The location of these arteries should be marked on the skin to assist with their preservation following elevation of the flap.
Following the incision, the pericranium is elevated to the level of the midline and down to the temporal line. At this point, an endoscope is used to assist in further flap elevation. Remaining within the areolar layer, the dissection is carried anteriorly until the supraorbital and supratrochlear arteries are localized.
The prior Doppler evaluation allows for the pedicle to be narrowed to approximately 3cm in diameter, bordered by the supraorbital and supratrochlear arteries. External landmarks can also be used to localize these vessels, such that the medial canthus is generally a landmark for the medial border and a 3cm lateral margin is generally sufficient to capture both arteries within the flap. Flap creation then proceeds 5cm posteriorly along the superior temporal line within the subgaleal plane.
Once the galea has been completely raised from the future pericranial flap, electrocautery can be used to incise the pericranium. The flap will span from midline medially to the superior temporal line laterally, and from the 3cm pedicle anteriorly to 5cm posterior to the coronal incision. Following the incision of the pericranium, the flap is raised from the frontal bone.
Transposition of the flap using the endoscopic technique is useful for repair of endonasal defects in the skull base. Therefore, a 1cm glabellar incision is the suggested method to transpose the flap. A diamond burr is used to create an osteotomy from the ipsilateral medial canthus to the contralateral medial canthus. Next, subperiosteal dissection connects the glabellar incision to the medial pericranial dissection.
The flap can then be transposed into the endonasal compartment. Along the endonasal compartment, a collagen graft should be placed within the dural defect. The pericranial flap can then be rotated onto the dural defect as an onlay to the collagen graft. Surgicel can then be packed onto the pericranial layer and fibrin glue applied to the surface. Gelfoam can then be placed on the fibrin glue layer followed by insertion of Merocel sponges. This multilayered vascular flap closure provides a robust watertight seal of the dural defect.
Complications
Complications of attempted skull base defect closure include headache, epistaxis, nasal crusting, abscess formation, meningitis, cerebritis, brain abscess, and seizures. Recurrence or formation of CSF leakage can occur if there is postoperative displacement of the closure material or poor wound healing, which are the most common complications during the postoperative period.
In the event of a recurrent CSF leak, lumbar drainage for 3 to 5 days should be attempted to permit postoperative healing at the site of the dural defect. If this maneuver fails, a re-exploration is reasonable.
Nasoseptal flap creation can result in loss of olfaction, related to the site and trajectory of the superior flap incision.
Postoperative Management
All dural closure techniques should be accompanied by appropriate prophylactic postoperative measures. These measures should be carefully discussed with the patient both before and after surgery to ensure compliance. These measures include avoidance of coughing, nose blowing and straining, and the use of stool softeners and open mouth sneezing.
Antibiotics, generally third-generation cephalosporins, are typically administered postoperatively. Saline and nasal antibiotic sprays are useful for mucosal healing. Patients should avoid aggressive physical activity and any activity that leads to elevations in intracranial pressure for 1 month after the operation.
Imaging evaluation, particularly with a CT scan, is routinely employed to detect excessive pneumocephalus or intracranial bleeding. Accumulation of a massive amount of pneumocephalus in the absence of obvious CSF leakage should alert the surgeon to discontinue lumbar CSF drainage and search for an occult CSF fistula that would require operative repair.
Otherwise, continuation of lumbar drainage will lead to reverse suctioning of air through the nose and dramatic worsening of the pneumocephalus. These cascades may cause acute neurologic deterioration and infection. A repeat operation with careful dural closure and skull base reconstruction using vascularized regional and free tissue-transfer flaps are often necessary.
Because delayed hydrocephalus after skull base surgery is possible, CSF leakage, if unresponsive to temporary lumbar drainage or definitive operative repair, may demand placement of a ventriculoperitoneal shunt.
If a nasoseptal flap repair has been performed, the gloved Merocel sinus packs (Medtronic, Minneapolis, MN) are removed on postoperative days 7 to 10.
Pericranial flap techniques benefit from the use of a head wrap until postoperative day 2 to decrease the edema adjacent to the cranial incision.
Routine endoscopic endonasal evaluation during the postoperative period is advantageous. This philosophy permits assessment of epithelialization and removal of debris to promote mucosal healing.
Pearls and Pitfalls
- I always conduct the opening with the closure in mind. Indiscriminate use of wide exposures should be avoided.
- Operator’s patience during closure and meticulous attention to detail are warranted. After a long case, it is often tempting to rush through the closure. This temptation can spoil the results of a great operation via postoperative leakage of CSF.
Contributors: Jonathan Ting, MD, MBA and Benjamin K. Hendricks, MD
References
Snyderman C, Gardner P (eds). Master Techniques in Otolaryngology-Head and Neck Surgery: Skull Base Surgery. Wolters Kluwer, 1st edition, 2014.
Tabaee A, Placantonakis DG, Schwartz TH, et al. Intrathecal fluorescein in endoscopic skull base surgery. Otolaryngol Head Neck Surg. 2007;137(2):316–320.
Please login to post a comment.