Paraclinoid Aneurysm
This is a preview. Check to see if you have access to the full video. Check access
Clip Ligation of a Small Ophthalmic Artery Aneurysm
Ophthalmic artery aneurysms arise from the supraclinoid internal carotid artery (ICA), and more specifically, the ophthalmic segment of the supraclinoid ICA. This segment encompasses the ICA from the distal dural ring to the posterior communicating artery.
Ophthalmic segment aneurysms are intradural. They include ophthalmic artery aneurysms, as well as superior hypophyseal aneurysms and their rare variants such as those on the dorsal and ventral surfaces of this ICA segment. The term paraclinoid aneurysm is nonspecific and includes ophthalmic segment aneurysms in addition to transitional or distal cavernous ICA aneurysms on the cavernous or clinoidal segments of the ICA.
Ophthalmic artery aneurysms project superiorly and arise from the ICA as it enters the subarachnoid space. These aneurysms account for no more than 5% of all intracranial aneurysms, and are often bilateral (mirror) and found in middle-aged women.
Usually found as unruptured, these aneurysms can be quite large at the time of their diagnosis. A thorough radiographic evaluation is warranted because this aneurysm subtype can be difficult to treat microsurgically. Their involvement with the optic apparatus, large size, and relationship to the anterior clinoid process present unique challenges for their proximal control, adequate neck exposure, and clip ligation.
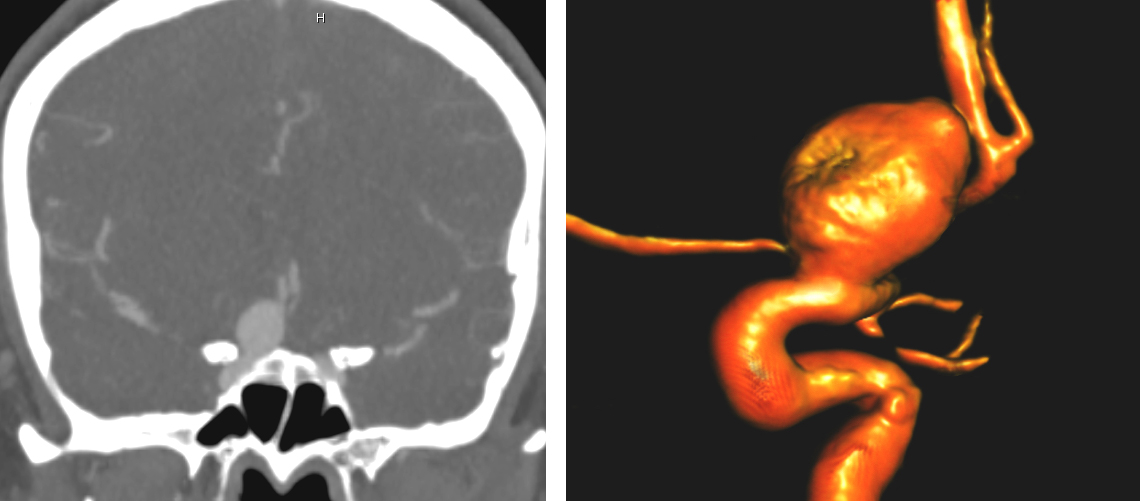
Figure 1: A large right-sided ophthalmic aneurysm is demonstrated. Note the intimate relationship of the anterior clinoid process (left) and the ophthalmic artery’s origin (right).
Indications for the Procedure
Potential management options for ophthalmic aneurysms include observation, endovascular treatment, microsurgical clip ligation, and carotid occlusion with or without bypass. Although ruptured aneurysms require treatment, unruptured small asymptomatic aneurysms can undergo observation with serial imaging. The International Study of Unruptured Intracranial Aneurysms (ISUIA) found that anterior circulation aneurysms smaller than 7 mm carry a rupture rate far less than 1%. In this situation and for middle age or older adults, observation is a very reasonable option.
For young patients with aneurysms approaching 7 mm, a treatment plan should be individualized based on the patient’s history of subarachnoid hemorrhage, family history of cerebral aneurysms, and ultimately the patient’s preference. Symptomatic lesions with mass effect on the optic nerve and those larger than 7 mm in patients with a significant life expectancy generally warrant treatment.
These aneurysms have an intimate relationship to the anterior clinoid process and optic apparatus, and their proximal neck may complicate safe application of a clip across the entire neck. Consequently, some operators consider microsurgery technically risky and endovascular therapy the preferred option for suitable aneurysms. Small-neck aneurysms are ideal for coiling and stent/balloon-assisted embolization; these treatments have expanded the endovascular applications. Furthermore, flow-diverting stents have facilitated treatment of complex and giant aneurysms previously considered for microsurgery.
The endovascular literature reports treatment morbidity for these aneurysms in the range of 0-10%. The addition of stent-assisted coiling has been found to be relatively as safe with reference to visual outcomes. The rate of complete lesional occlusion varies based on the size and complexity of the aneurysm, and recurrence rates approach 20%. Visual outcomes have shown improvement or stabilization in the majority of patients.
Microsurgery for ophthalmic artery aneurysms generally carries a morbidity rate of 5- 20% and mortality of 5%. Although the complications are generally higher when compared with most endovascular series, microsurgery offers the potential advantage of optic apparatus decompression among symptomatic patients.
Microsurgery also offers the advantage of durability, with a long-term recurrence rate usually less than 5 %. Although endovascular intervention is likely safer for many patients, microsurgery is an option for those with symptomatic optic apparatus compression, young age, and an aneurysm location minimally complicated by skull base anatomy (clinoidal or carotid cave aneurysms).
Preoperative Considerations
Evaluation of ophthalmic segment aneurysms includes CT or catheter angiography. Magnetic resonance angiography may also be used as an adjunct assessment modality, but not as the sole radiographic study. Determining the intradural location of the aneurysm is important because extradural aneurysms are not associated with a risk of subarachnoid hemorrhage, and therefore rarely warrant treatment.
Computed tomography angiography (CTA) can be especially helpful in defining the exact location of the aneurysm with respect to bony landmarks such as the anterior clinoid process. This imaging modality can reliably estimate the intradural extent of the aneurysm and need for treatment.
Bony imaging (CT and CTA) can also evaluate the presence of a medial clinoid process that fuses with the anterior clinoid process, completing a bony ring around the ICA, complicating the clinoidectomy procedure. Additional diagnostic tests should include preoperative visual field tests if the aneurysm is in contact with the optic nerves.
Although the anatomic definition of the ophthalmic segment is useful in that it excludes extradural aneurysms, it can be difficult to reliably and consistently determine if all aneurysms are definitively intra- or extradural. Because up to 8% of ophthalmic arteries can arise extradurally, an ophthalmic artery aneurysm in this instance can be extradural, transitional (spanning both sides of the distal dural ring), or intradural, based on the exact location of its dome. The optic strut is a reliable marker of the proximal dural ring (the endpoint of the cavernous segment on CTA.)
In patients with large, calcified, or morphologically complex lesions, clip ligation may be technically challenging or impossible, and carotid Hunterian ligation with or without distal bypass may be an option. For these patients, a balloon test occlusion (BTO) can determine the surgical risk, although this test is not 100% reliable for estimating the risk of postoperative ischemia after Hunterian ligation.
Carotid ligation without BTO could result in infarction among ~25% of patients. The addition of BTO with blood flow studies has decreased this complication to less than 10%, and newer adjuncts such as hypotensive challenge have shown further improvements at the cost of an increased incidence of false positive results. Therefore, if carotid occlusion is contemplated, I strongly advocate for a high-flow radial artery bypass graft to revascularize the middle cerebral artery territory.
More recently, endovascular flow diversion has become another effective treatment route for these large, complex aneurysms. Although long-term data are not yet available, its efficacy in select patients is proven. This has led to a very limited number of patients with ophthalmic artery aneurysms (young patients or those presenting with mass effect on the optic nerve) who are referred for microsurgical intervention.
Operative Anatomy
The operative anatomy of the region is complex and its mastery in the microsurgical laboratory is required.
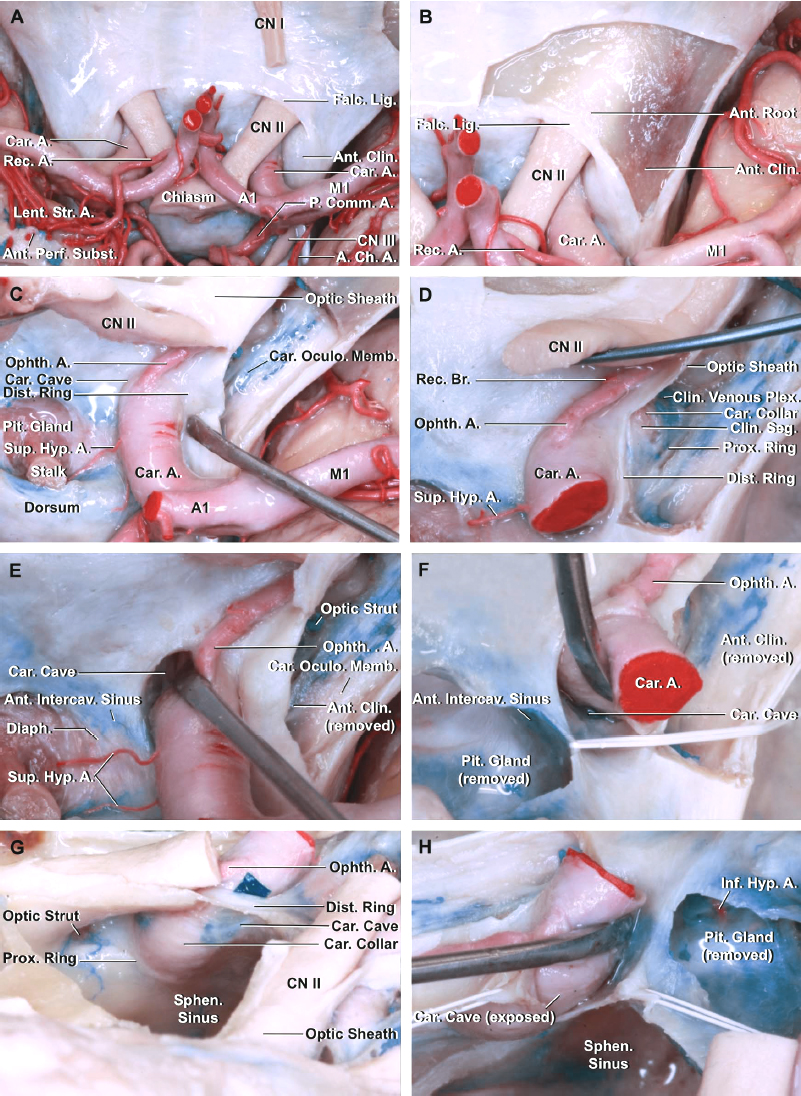
Figure 2: A lateral view of the right internal carotid artery with the anterior clinoid process and dura propria of the cavernous sinus removed. Note the location of the ophthalmic artery, which is often covered by the optic nerve and the anterior clinoid process. The ophthalmic segment begins at the distal dural ring (upper ring) and ends at the origin of the posterior communicating artery (not visible in this picture)(image courtesy of AL Rhoton, Jr.)
The intracranial ICA is approximately 4-6 mm in diameter and carries a blood flow of ~300 cc/min. Evidence of carotid artery classification systems dates back to the 17th century and has generally evolved into anterograde classification systems
Van Loveren and colleagues have defined seven anterograde segments of the ICA based on the surrounding anatomy. These segments include: cervical (C1), petrous (C2), lacerum (C3), cavernous (C4), clinoidal (C5), ophthalmic (C6), and communicating (C7).
Although the anatomy of the C6 segment is the most relevant to ophthalmic artery aneurysms, the distal cavernous (C4) and clinoidal (C5) segments are also important, not only for the relevance of the adjacent bony anatomy, but also for transitional and cavernous aneurysms that may be difficult to identify as intradural aneurysms. The cavernous segment begins at the superior margin of the petrolingual ligament and extends to the proximal dural ring near the anterior clinoid process.
Branches of the cavernous segment include the meningohypophyseal trunk, the inferolateral trunk, and the capsular artery. The clinoidal segment is a short segment without branches that is deep to the anterior clinoid process and bordered by the proximal and distal dural rings. The ophthalmic segment begins at the distal dural ring and ends at the origin of the posterior communicating artery. It owns two branches, the ophthalmic and superior hypophyseal arteries, and the latter can be a group of small perforators rather than a single vessel.
Click here to view the interactive module and related content for this image.
Figure 3: The anatomy around the paraclinoid carotid is depicted (A). Note the relationship of the anterior clinoid process to the ICA and the optic nerve. The dura lining the roof of the optic canal and anterior clinoid has been removed. The falciform ligament extends along the posterior edge of the anterior root of the lesser wing and above the optic nerve to blend medially into the dura mater covering the planum sphenoidale (B). The optic nerve and chiasm have been mobilized to expose the pituitary stalk, ophthalmic artery, and superior hypophyseal artery (C). The anterior clinoid process has been removed to expose the carotid oculomotor membrane formed by the dura lining the lower margin of the clinoid that separates the clinoid from the oculomotor nerve and extends medially to form the proximal dural ring. The clinoidal segment of the ICA between the proximal and distal dural rings is exposed (D). The right carotid artery is retracted to show the carotid cave (E and F). The roof of the sphenoid sinus has been removed through this oblique anterosuperior view to expose the medial side of the right ICA. A green piece has been placed into the carotid cave (G). The distal dural ring has been divided and the dural flaps retracted with white silk to expose the carotid cave (H)(images courtesy of AL Rhoton, Jr.)
Bony and dural anatomy complicates the surgical exposure of ophthalmic aneurysms. As the cavernous ICA takes an anterior path after it arises from the lacerum segment, it runs in the carotid groove/sulcus and makes a nearly complete change in direction, initially turning upward and then posteriorly as it exits the cavernous sinus, passing under the anterior clinoid process and entering the intradural space.
The anterior clinoid process covers the most distal extradural segment of the ICA and frequently covers the base of ophthalmic artery aneurysms. This process is the posteromedial extension of the lesser wing of the sphenoid bone and averages approximately 8x4 mm. It has a superior-medial root that covers the optic canal and joins the planum sphenoidale, and an inferior root that forms the optic strut and attaches to the tuberculum sella of the basisphenoid.
A clinoidectomy requires disconnection of all three attachments of the clinoid process. The technical nuances of clinoidectomy are described in the Intradural and Extradural Clinoidectomy chapters.
Although the majority of patients own no bony attachment to the posterior end of the anterior clinoid process, a small number of patients have a piece of bone bridging from the posterior clinoid process to the middle clinoid process. The latter is a bony prominence in roughly one-fourth of patients that on rare occasions completely encircles the ICA; an extradural fracture of the process in this instance could result in severe ICA injury. Preoperative CTA can be informative to avoid this complication.
The dura surrounding the anterior clinoid process is also complex. The cavernous dura is comprised of two layers, the thick dura propria and a thinner inner dura. The inner dura covers the lateral cavernous neural elements, contributes to the sheath of the occulomotor nerve, and continues anteriorly to cover the inferior surface of the anterior clinoid process. It also surrounds the ICA, forming the proximal dural ring, which is often incompetent and does not attach to the adventitia of the artery.
The dura propria continues on top of the process, combining with the dura of the planum sphenoidale and the ICA, forming the distal dural ring. The distal dural ring is slanted downward from an anterolateral to posteromedial direction. This ring is usually not circumferential, but it is strongly adherent to the adventitia of the carotid artery; dissection of the ring from the artery is dangerous and often impossible.
Although the lateral and superior portions of the distal dural ring directly extend from the clinoid and cavernous dura to the carotid adventitia, the posterior medial dura turns downward before attaching to the carotid wall, creating a potential space referred to as the carotid cave. Cave dimensions vary, but one anatomic study found the cave present in 95% of specimens with a depth of ~2.5 mm and a length of 1 cm. This pouching of the dura can house a cavernous aneurysm that may potentially have an extradural neck, but an intradural dome.
Classification of Paraclinoid/Ophthalmic Artery Aneurysms
Multiple classification systems exist for ophthalmic segment aneurysms. Most of these classifications include peri/ophthalmic artery, superior hypophyseal artery, and clinoidal and cavernous aneurysms. Lawton classified the intradural ICA aneurysms simply as ventral or dorsal variants:
- Ophthalmic artery aneurysms: These aneurysms typically arise just distal to the ophthalmic artery and point superior or superomedially, although they can also rarely point laterally. These aneurysms elevate the optic nerve and entrap it against the falciform ligament, often producing a visual field defect (an inferomedial quantrantanopsia).
- Superior hypophyseal aneurysms (SHAs): These aneurysms project inferomedially along the path of blood flow into the suprasellar region. Depending on the location of the superior hypophyseal artery, such aneurysms can point superiorly and be referred to as suprasellar SHAs, as opposed to parasellar SHAs. Large aneurysms can elevate the optic chiasm and cause a bitemporal hemianopsia similar to those of a pituitary macroadenoma.
- Variant aneurysms: Intradural ophthalmic segment aneurysms that are not associated with an arterial branch have been referred to as dorsal or ventral ICA aneurysms. Dorsal variant aneurysms are thought to be secondary to hemodynamic shear stress, are often blister pseudoaneurysms, and can be very fragile. Ventral aneurysms include those both proximal and distal to the superior hypophyseal artery. The term carotid cave aneurysm is typically used to describe proximal ventral carotid aneurysms that project downward into the dural recess at the inferomedial portion of the distal dural ring (carotid cave). The term ventral carotid aneurysm is typically used to describe aneurysms distal to the superior hypophyseal artery, but not associated with the posterior communicating artery.
- Clinoidal aneurysms: These extradural aneurysms have an anterolateral variant, potentially causing optic apparatus compression and visual loss, and a medial variant, potentially eroding into the sella and posing a risk of apoplexy or severe epistaxis.
CLIP LIGATION OF OPHTHALMIC ARTERY ANEURYSMS
With all paraclinoid aneurysms, the patient’s neck should be sterilely prepared and marked for dissection if proximal control becomes necessary. Neck dissection is often unnecessary for small unruptured aneurysms, but often prudent for aneurysms that are ruptured or large.
Proximal control can be achieved by temporary clipping of either the ICA or both the external carotid artery (ECA) and the common carotid artery (CCA). Clamping the ECA and the CCA avoids potential injury to the ICA. Despite proximal ICA occlusion in the neck, back bleeding from the posterior communicating and ophthalmic arteries can be brisk if intraoperative rupture occurs.
A pterional craniotomy is sufficient for adequate exposure of almost all ophthalmic artery aneurysms. An orbitotomy does not usually provide any significant advantage. The supraorbital craniotomy through the eyebrow incision is a very reasonable route for uncomplicated aneurysms.
The patient’s head position during surgery for these medially situated aneurysms demands less neck rotation (15-20 degrees) to allow the surgeon to look under the optic nerve after the clinoidectomy. Slightly less head extension lessens the steep viewing trajectory under the anterior clinoid process.
An intradural clinoidectomy is optional given the presence of a tenuous underlying aneurysm prone to premature rupture. I perform the clinoidectomy intradurally for ruptured aneurysms and extradurally for unruptured aneurysms.
INTRADURAL PROCEDURE
Initial Exposure and Intradural Clinoidectomy
Before dural opening and after completion of the craniotomy, the medial sphenoid wing is resected extradurally to the level of the lateral clinoid process. After a standard dural opening, the anterior limb of the Sylvian fissure is split to gain access to the ICA, anterior clinoid process, and optic nerve.
Opening of the arachnoid layers proceeds in the standard fashion from distal to proximal, identifying the distal ICA and posterior communicating artery to obtain distal control if necessary. Microdissection then continues proximally until the neck of the aneurysm on the ICA is identified. Care should be taken to avoid aggressive frontal lobe retraction, as the aneurysm may be adherent to the orbitofrontal cortex; manipulation may lead to premature rupture. The anterior clinoid process frequently hides the ophthalmic artery origin and most likely covers the anterior aneurysm neck and more proximal ICA. Increased exposure is achieved through intradural anterior clinoidectomy.
Distal Ring Dissection
After the clinoidectomy, the dissection involves release of the dural leaflets of the optic sheath and distal dural ring. The optic nerve partially blocks the origin of most ophthalmic artery aneurysms. In order for the surgeon to visualize the corresponding ICA segment, the optic nerve must be mobilized medially and the ICA mobilized laterally.
The optic nerve is then untethered and gently mobilized by sectioning the falciform ligament. A fine right-angled blunt-tipped dissector defines the space above the optic nerve under the ligament and a right-angled diamond knife safely cuts this leaflet, fully unroofing the optic nerve.
The distal dural ring tethers the ICA medially to the optic strut and dura of the optic sheath. Drilling the strut releases this dura, creating a soft tissue plane to cut the dura surrounding the distal dural ring.
The initial dural cut can be made laterally on the ICA and proceeds superomedially toward the axilla between the optic nerve and the ICA. If the tips of the scissors are impeded from further progress along the medial aspect of the ICA, the optic strut may have been insufficiently drilled. At this point, proximal ICA exposure may be sufficient to safely evaluate the aneurysm neck and apply a permanent clip. Most aneurysms do not require further dissection before their clip ligation.
If necessary, further mobilization of the ICA is possible through continued drilling of the bone and sectioning of the distal dural ring. Once the medial dural ring is released, the remaining ring can be incised from the lateral initial incision in an inferomedial direction. Full circumferential dissection is often neither possible nor necessary. However, near circumferential dissection can be a vital maneuver for exposure of superior hypophyseal or very proximal ophthalmic artery aneurysms.
Aneurysm Dissection and Clip Application
Small ophthalmic artery aneurysms are technically easy to clip after an extradural clinoidectomy. Larger aneurysms present technical challenges, especially if their neck is extending into the carotid cave.
Figure 4: After extradural removal of the left anterior clinoid process, opening of the falciform ligament and mobilization of the ICA, the origin of the ophthalmic artery and the neck of the small aneurysm should be readily visible. Not infrequently, the artery’s origin seems to be involved at the neck of the aneurysm, requiring reconstruction.
The ophthalmic artery’s origin is dissected from the proximal neck, and the surgeon must take care to gently retract the ICA laterally rather than the optic nerve medially. Temporary clipping of the cervical ICA greatly aids in this dissection maneuver by softening the aneurysm sac. However, most small unruptured aneurysms can be clip ligated without proximal control.
Figure 5: Although small ophthalmic artery aneurysms can be ligated with a straight or curved clip, I prefer to use side-angled clips for all such aneurysms regardless of their size or complexity (including partially atherosclerotic or thrombotic aneurysms). Side-angled clips orient the blades parallel to the long axis of the parent ICA and allow efficient neck closure without causing accordion-like shortening of the carotid trunk. A perpendicular clipping technique across the ICA leads to partial neck closure, hemodynamic turbulence within the sac, and potentially intraoperative rupture.
Figure 6: Once the aneurysm is clip ligated and fluorescence imaging confirms a lack of intrasaccular flow, the dome can be punctured to ensure complete obliteration and optic apparatus decompression. Fluorescence angiography can occasionally produce false negative results, and so could occur with microdoppler ultrasonography.
There is a distinct difference in the technical complexity of ligation for superiorly projecting and anteromedially projecting ophthalmic aneurysms. Although the former are relatively straightforward, the latter are hidden under the optic nerve and often require a tandem clipping technique: a fenestrated clip around the ICA can often be used to close a remnant.
Whereas ophthalmic artery aneurysms are often well visualized, superior hypophyseal aneurysms project medially away from the surgeon, with the ICA blocking any substantial view of the neck. These aneurysms usually require an angled fenestrated clip with the ICA within the fenestration and the clip blades pointing toward the distal dural ring. Because the superior hypophyseal arteries are often very proximal, the tips of the clip blades must extend up to or past the distal dural ring to completely close the neck. If the ring is not circumferentially dissected, the clip blades will remain partially splayed open and the aneurysm sac will continue to fill.
Visualization of the entire aneurysm neck during clip application is often impossible, and clip deployment proceeds with visualization of one blade and often inspection of the other only after the clip is applied. Depending on the anatomy, one or several angled or right-angled fenestrated clips may be necessary. Inspection should reveal no perforator injury.
Variant aneurysms can often be handled using similar techniques. Dorsal ophthalmic segment aneurysms are clip ligated similar to ophthalmic artery aneurysms, and ventral aneurysms are treated similarly to SHAs.
Large and Complex Ophthalmic Artery Aneurysms
Large and giant aneurysms require the use of complete flow arrest (aneurysm trapping) or suction-decompression technique for their decompression, manipulation and clip placement while preventing undue retraction on the optic apparatus. The purpose is not only to prevent intraoperative rupture, but also to obtain adequate neck visualization and reliable neck closure.
With large to giant aneurysms, aneurysmal decompression using the retrograde suction-decompression technique can be lifesaving. With this technique, endovascular inflation of a balloon in the cervical ICA is followed by temporary clip occlusion of the distal ICA within the operative field. Retrograde suction of the blood using a balloon catheter in the neck provides dramatic deflation and clip reconstruction of the patent ICA.
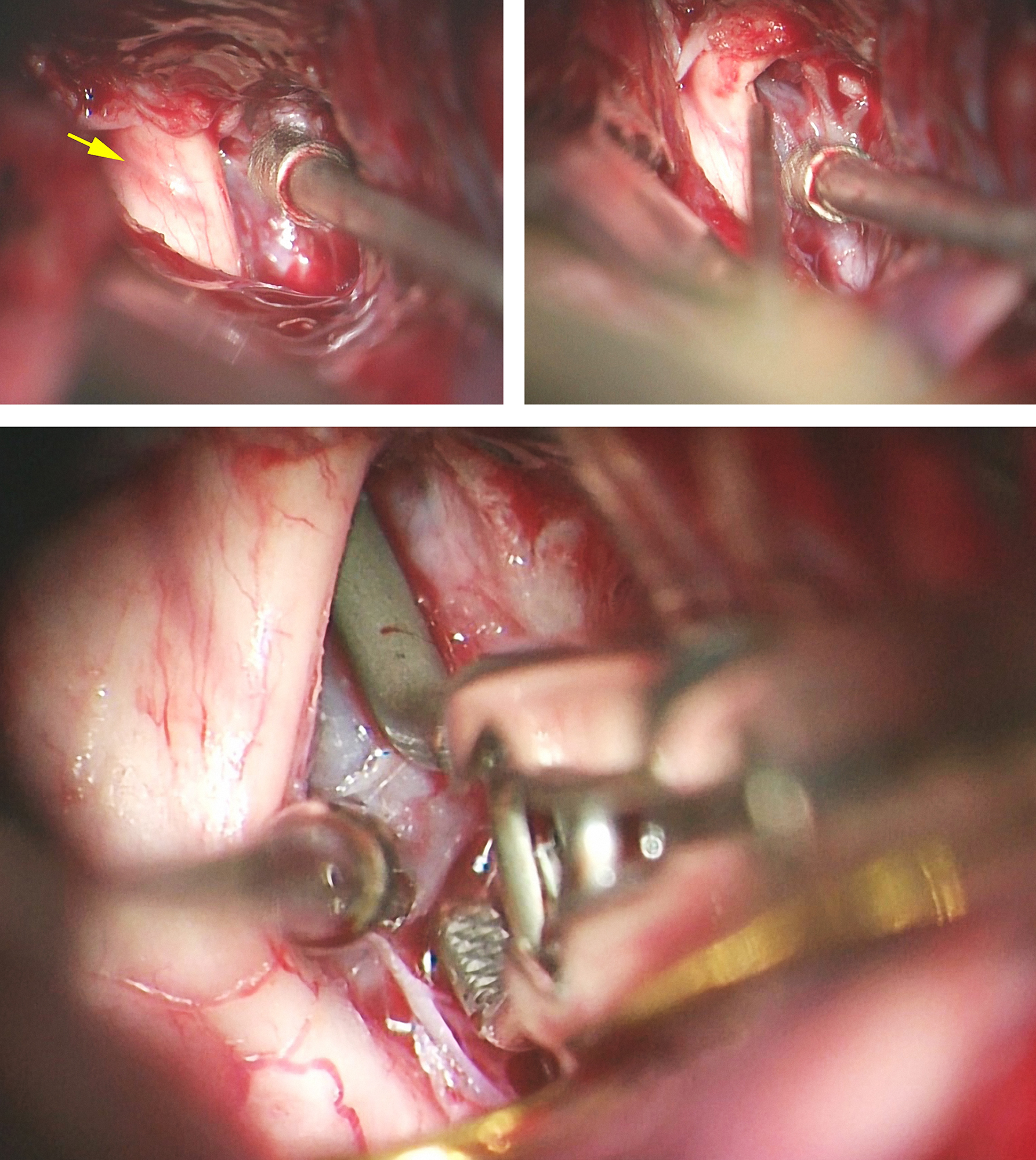
Figure 7: A large right-sided ophthalmic artery aneurysm (see Figure 1) was clip ligated using the suction-decompression technique. Note the discoloration on the optic nerve from the mass effect of the aneurysm (left upper image, yellow arrow) after the falciform ligament was incised. The suction-decompression technique provided the much needed deflation of the aneurysm so I could identify the anterior neck (right upper image) and apply the permanent clip (lower image).
Simple side-angled clips will leave residual neck medially on large or complex aneurysms. In these instances, the first clip should be rotated medially to close the medial neck while a second understacked clip can grab the lateral neck. In addition, tandem clipping can be quite effective in large aneurysms that frequently harbor thick walls.
Partially thrombosed aneurysms with atherosclerotic necks can force the clip blades onto the parent ICA. To avoid ICA stenosis/compromise, I incise the sac and perform a thrombectomy to allow safe placement of the permanent clips. An ultrasonic aspirator aids in timely removal of the sac contents. Obviously, temporary ICA trapping is necessary.
Both microdoppler ultrasonography and fluorescence videoangiogram can quickly evaluate residual aneurysm filling and arterial stenosis. For simple aneurysms, this imaging modality is adequate. For complex aneurysms or questionable findings on videoangiogram, an intraoperative catheter angiogram is definitive. Final inspection should ensure complete obliteration of the aneurysm, decompression of the optic nerve, and a lack of perforator compromise.
Large Ophthalmic Artery Aneurysm: Intradural Clinoidectomy and Suction Decompression Technique
Other Considerations
The risk of visual compromise after clip ligation of complex ophthalmic artery aneurysms is not small. The clip should not compress or rotate the optic nerve that is especially intolerant of torsion. Delayed visual worsening indicates a need for re-exploration to assure the clip is not displaced, causing compression.
Closure and Postoperative Considerations
The closure and postoperative care are generally no different than those of other anterior circulation aneurysms. If the sphenoid sinus was entered during an anterior clinoidectomy, its meticulous packing is important to prevent a cerebrospinal fluid fistula. Any small defect can be easily obliterated with a small piece of temporalis muscle.
Pearls and Pitfalls
- A thorough understanding of the anterior skull base anatomy is the foundation for safe exposure of the paraclinoid vascular lesions.
- Even minor manipulations of the compromised optic nerve can lead to blindness. Large and giant aneurysms are daunting to handle without generating new visual deficits. Flow diversion stents should be strongly considered in some cases.
Contributor: Chris S. Taylor, MD
References
Batjer HH, Kopitnik TA, Giller CA, Samson DS. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80(4): 650–658
Joo W, Funaki T, Yoshioka F, Rhoton AL. Microsurgical anatomy of the carotid cave. Neurosurgery. 2012; 70(2 Suppl Operative): 300–311; discussion 311–312
Kim JM, Romano A, Sanan A, van Loveren HR, Keller JT. Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery. 2000; 46(3): 670–680; discussion 680–682
Korosue K, Heros RC. “Subclinoid” carotid aneurysm with erosion of the anterior clinoid process and fatal intraoperative rupture. Neurosurgery. 1992;31(2): 356-359; discussion 359-360
Samson DS, Batjer HH. Aneurysms of the anterior carotid wall (ophthalmic). Chapter 4 in: Intracranial Aneurysm Surgery: Techniques. Futura Publishing 1990
Please login to post a comment.