Anterior Choroidal Artery Aneurysm
This is a preview. Check to see if you have access to the full video. Check access
Clip Ligation of an Anterior Choroidal Artery Aneurysm
Please note the relevant information for patients suffering from brain aneurysm is presented in another chapter. Please click here for patient-related content.
Aneurysms of the posterior wall of the internal carotid artery (ICA), namely the anterior choroidal artery (AChA) and posterior communicating artery (PCoA) aneurysms, represent the most common site for intracranial aneurysms (up to 35% of total aneurysms.)
The AChA arises a few millimeters distal and slightly lateral to the origin of the PCoA from the supraclinoid ICA. It has a slightly smaller caliber, can immediately branch after its origin, and supplies very important territories, such as the posterior limb of the internal capsule, basal ganglia, optic apparatus, cerebral peduncle, lateral geniculate body, and limbic system.
There is minimal collateral support for this vessel, and its preservation is of paramount importance during clip ligation of posterior carotid wall aneurysms. The distal ends of the blades should not compromise the integrity of this vessel, which is often serpentine and hidden, draping over the AChA aneurysm dome.
Most AChA aneurysms present with suprasellar and ambien cistern subarachnoid hemorrhage (SAH.) They rarely present with intracerebral/intaventricular hemorrhage or oculomotor nerve dysfunction.
In this chapter, I will describe the operative techniques for clip ligation of AChA aneurysms. The similar procedure for posterior communicating artery aneurysms is described in its own dedicated chapter. I have abbreviated the relevant content here and encourage you to refer to the chapter on PCoA aneurysms before proceeding with the following discussion.
Indications for Surgery
Modern treatment options for AChA aneurysms include observation, endovascular treatment, or microsurgical clip ligation. Observation is a reasonable option for small unruptured aneurysms or larger ones in older patients (>75 years old.) Patients with underlying medical problems that affect their survival may also be treated conservatively.
As with PCoA aneurysms, patients with significant life expectancy should be considered for more aggressive intervention than those with other small anterior circulation aneurysms. Aneurysms chosen for observation should be reconsidered for intervention if they increase in size or cause an oculomotor nerve palsy.
The AChA frequently emerges from the neck of the aneurysm. This neck configuration complicates endovascular therapy and places the small-caliber AChA at risk during coil deployment. Thus, microsurgical clipping continues to play an important role in the management of AChA aneurysms that require relatively minimal microdissection or brain retraction.
Calcified aneurysms, identified on CTA, are best managed via endovascular methods because clip ligation has a reasonable likelihood of compromising the origins of the AChA and the ICA by the thick collapsed walls of the parent vessels after clip deployment.
Preoperative Considerations
Initial radiologic evaluation of these aneurysms generally includes either a conventional catheter arteriography or CT/MR angiography. CT angiography (CTA) has become the mainstream initial study for evaluation because of the fine vascular detail it provides. Moreover, it shows the surrounding skull base anatomy, including the location of the anterior and posterior clinoid processes, with respect to the aneurysm neck.
Bone windowing of a CT scan can reveal the presence of a middle clinoid process, which is relevant when the surgeon is preparing for proximal control. A rare subset of these aneurysms project laterally into the temporal lobe. This anatomic variation warns the operator against early temporal lobe retraction in the setting of a ruptured aneurysm until proximal control is reliably established.
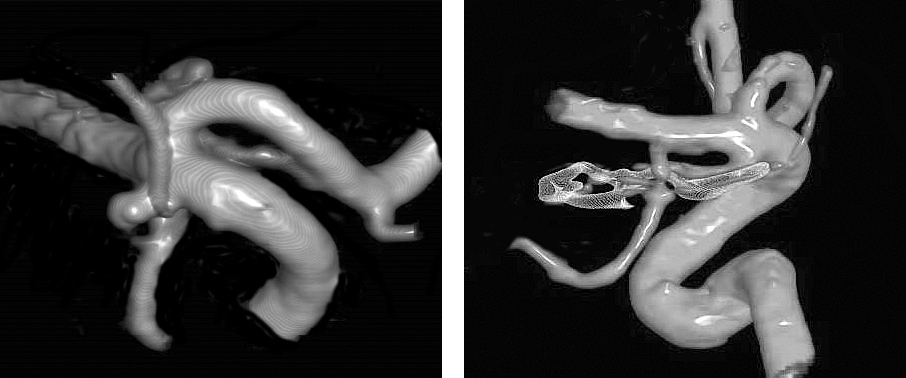
Figure 1: A typical AChA aneurysm is shown originating from the junction of the ICA and AChA (left image). The aneurysm arising from the posterior carotid wall, just distal to the PCoA origin is considered an AChA aneurysm and is typically more laterally projecting than PCoA aneurysms. Clip ligation is effective for complete exclusion of the aneurysm while maintaining the patency of the AChA (right image).
Intraoperative neurophysiologic monitoring, including somatosensory and motor evoked potentials, can monitor the patient’s tolerance to temporary occlusion and warn the surgeon about potentially invisible compromise of the perforating vessels after clip ligation. This monitoring strategy is especially beneficial for the AChA artery and its associated perforating vessels that supply the motor system.
Operative Anatomy
The AChA arises slightly more laterally than the PCoA from the posterior wall of the supraclinoid ICA. Identification of both vessels at the posterior carotid wall is important before operative manipulation begins.
The AChA may consist of up to three branches. It courses through the crural cistern en route to its entry point into the temporal horn of the lateral ventricle, providing several critical perforators along its way. Therefore, the AChA is divided into cisternal and plexal segments.
The AChA classically arises just distal to the PCoA, but it can occasionally arise more distally, either from the ICA bifurcation or the M1 segment. It can even emerge from the PCoA itself. Both the PCoA and AChA project posteriorly and medially from the dorsal aspect of the ICA. The anterior-to-posterior surgical view of the pterional approach places these vessels coursing away from the surgeon’s line of sight. All the surgeon sees is a knuckle of the very proximal artery at its origin before it becomes hidden behind the ICA.
Click here to view the interactive module and related content for this image.
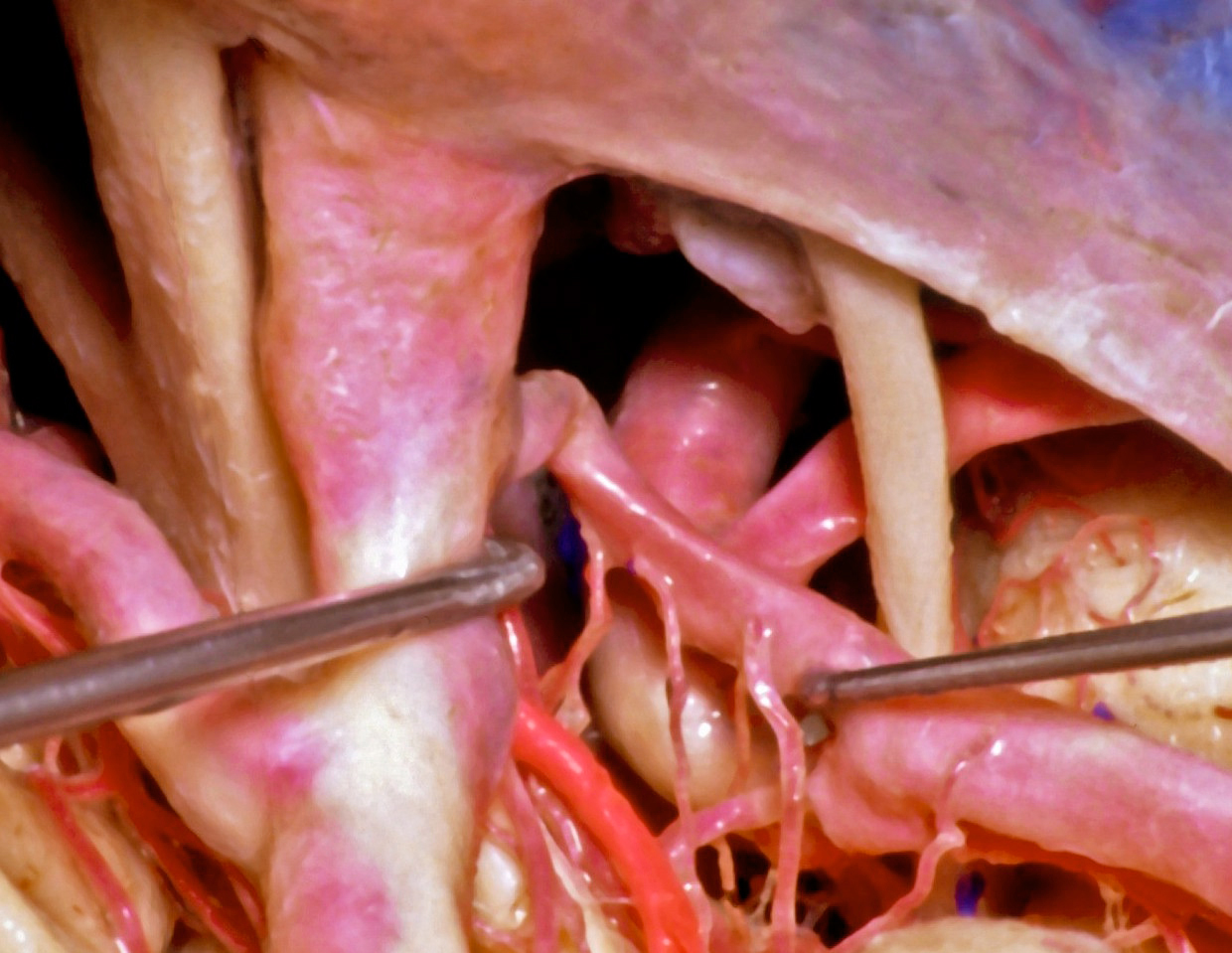
Figure 2: A lateral nonoperative view across the posterior ICA demonstrates the anatomic relationships between the PCoA (mobilized with the right dissector,) its perforating vessels, and the AChA (in bright red.) The oculomotor nerve is visible (image courtesy of AL Rhoton, Jr.)
Figure 3: A view of the AChA (bright red) through the right transventricular transchoroidal approach is shown. Note the edge of the tentorium and the oculomotor nerve for anatomic orientation (image courtesy of AL Rhoton, Jr.)
There are inconsistent anastomoses between the PCoA and AChA along the lateral geniculate body. This finding explains the variability in the size of the ischemic territories caused by AChA occlusion.
MICROSURGICAL CLIP LIGATION OF ANTERIOR CHOROIDAL ARTERY ANEURYSMS
Please refer to the Cranial Approaches volume for description of the extended pterional craniotomy that is suitable for almost all AChA aneurysms. A posterolateral orbitotomy may be added for large aneurysms. In this section, I elaborate on the specifics of the pterional approach as it pertains to the exposure of this aneurysm.
The main objectives of patient positioning are to prevent the temporal lobe from obstructing your dissection of the aneurysm while exploiting gravity to move the frontal lobe away from the anterior cranial fossa. These maneuvers expand the posteromedial subfrontal operative corridor and are accomplished by extending the patient’s head and limiting its rotation to 20 degrees.
The sphenoid wing must be aggressively drilled until the superior orbital fissure is reached. The most medial extent of the wing is the most important because it covers the carotid cistern. If this last obstruction is not removed, substantially more frontal lobe elevation is needed to expose the cistern. The roof of the orbit is also drilled so that a flat operative trajectory over the orbit is available.
Figure 4: Schematic representation of the posterior subfrontal operative pathway (green arrow) toward the AChA aneurysm. This pathway is possible via a proximal Sylvian fissure split, leading directly to the anterior and lateral aspects of the supraclinoid ICA. Early exposure of the proximal ICA without temporal lobe retraction is necessary to avoid premature sac rupture.
INTRADURAL PROCEDURE
Initial Exposure
There are three main goals that must be accomplished before the aneurysm can be directly handled.
The first goal is a proximal Sylvian fissure split.
The second goal is mobilization of the frontal lobe and dissection of its arachnoid bands over the chiasm and floor of the frontal fossa.
The third goal is establishing proximal control over the supraclinoid ICA. The aneurysm must be exposed solely via frontal lobe retraction. The crux of the initial exposure is freeing up the posterior subfrontal lobe so that it can be safely and gently mobilized without any transmission of force to the aneurysm dome.
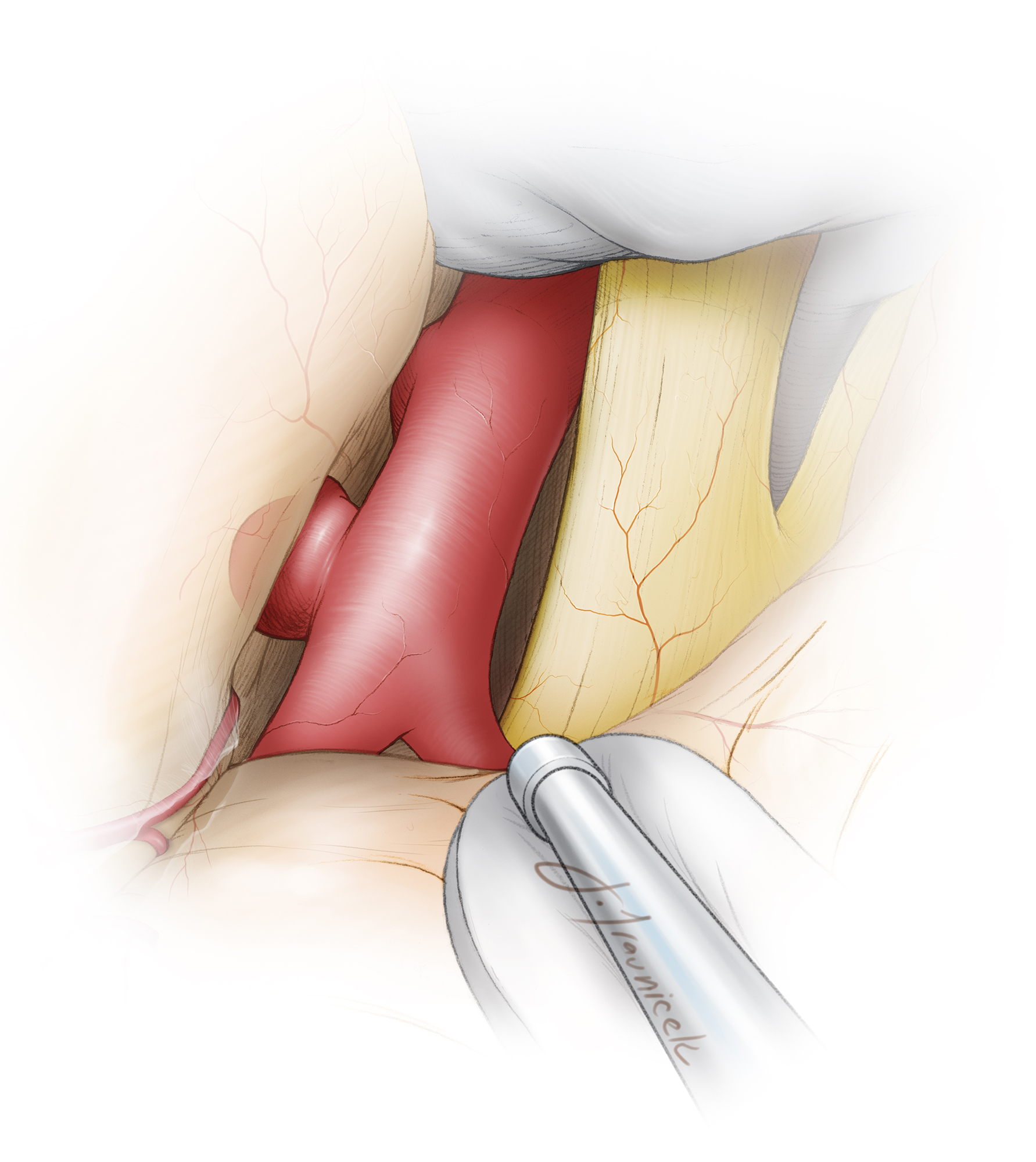
Figure 5: The initial intradural exposure is illustrated. Adequate sphenoid wing removal is evident when the dura can be mobilized flat over the orbital roof and along the anterior edge of the craniotomy.
The initial steps of the intradural procedure, including the exposure of the posterior carotid wall, are discussed in the Posterior Communicating Artery Aneurysm chapter. The following paragraphs specifically refer to AChA aneurysms.
Figure 6: The posterolateral wall of the left carotid artery is exposed. The origin of the PCoA is apparent only as a small knuckle a few millimeters inferior to the aneurysm sac. Temporary clipping of the ICA is often necessary, especially for ruptured aneurysms, during the next steps of neck dissection.
With the brain relaxed, the frontal lobe completely released, and proximal control secured, the focus of dissection can now shift toward the circumdissection of the neck, the most critical part of the operation. Application of the clip is a relatively straightforward affair. The creation of space for the clip blades is the challenge, and preclipping dissection requires surgical intellect and appreciation of three-dimensional (3D) anatomy based on a detailed study of preoperative images.
Aneurysm Dissection
The aneurysm sac and AChA travel away from the operator. As a result, only a small knuckle, representing the origin of the AChA, is visible. I prefer to use brief periods of temporary proximal ICA occlusion; I do not believe etomidate burst suppression is necessary during temporary proximal ICA occlusion if collateral support is robust via a functional circle of Willis. The loss of turgor in the aneurysm remarkably facilitates its neck dissection during high-risk maneuvers.
The neck dissection proceeds between this knuckle and the proximal neck of the aneurysm and proceeds distally along the lateral aspect of the ICA. Although this maneuver can be tedious, sharp dissection under high magnification will almost always find a plane between the AChA and the aneurysm neck.
Only a few millimeters of the aneurysm neck is isolated, just enough to place a clip blade. There is no need to dissect more of the artery or dissect out onto the aneurysm dome. If the AChA cannot be readily identified, the ICA can be gently retracted medially to allow the surgeon to explore the origin of the artery more distally along the neck.
Figure 7: The distal neck is the space between the ICA and the aneurysm; similar microsurgical principles mentioned above apply to this space as well. The AChA is sometimes adherent to the midbody or fundus of the aneurysm. Temporary proximal ICA clipping is especially beneficial to soften the aneurysm, allowing dissection of the AChA away from the neck without placing the AChA at risk. Once the proximal and distal necks have been dissected and the courses of the PCoA and AChA identified, definitive clipping of the aneurysm can commence.
With temporary ICA occlusion in place, I mobilize the proximal and distal neck and dissect the portion of the neck that is turning away from my line of sight, ensuring that I can see where the neck is turning around to form its medial border with respect to wall of the ICA. This maneuver is imperative because a lack of understanding of the 3D neck anatomy will lead the operator to apply the clip at a wrong angle away from the ICA axis, partially clipping the neck and precipitating an intraoperative rupture.
Completing dissection blindly using the clip blades and “guessing at the deeper aneurysm neck borders” are recipes for disaster. These novice maneuvers are a result of a nervous surgeon who is allowing his or her emotions to control microsurgery.
In ruptured aneurysm cases, the subarachnoid clot around the dome is left undisturbed until the aneurysm is secured. The dissection should be limited along the posterolateral wall of the supraclinoid ICA and the neck; the operator should not wander and unintentionally puncture or uncover the aneurysm dome. The sequence of dissection should proceed methodically from proximal to distal, revealing the knuckle of the PCoA origin, the proximal neck and AChA origin, the sac itself, the distal neck and then the distal section of the AChA away from the pathway of the clip blades. Next, the clip is deployed.
Clip Application
Most AChA aneurysms are small and have relatively narrow necks. I ligate these aneurysms with a bayoneted simple straight clip, angled perpendicular to the ICA axis with the tips pointing laterally. Although an accordion effect can potentially cause stenosis of the ICA lumen, this is less of a concern if the aneurysm has a narrow neck.
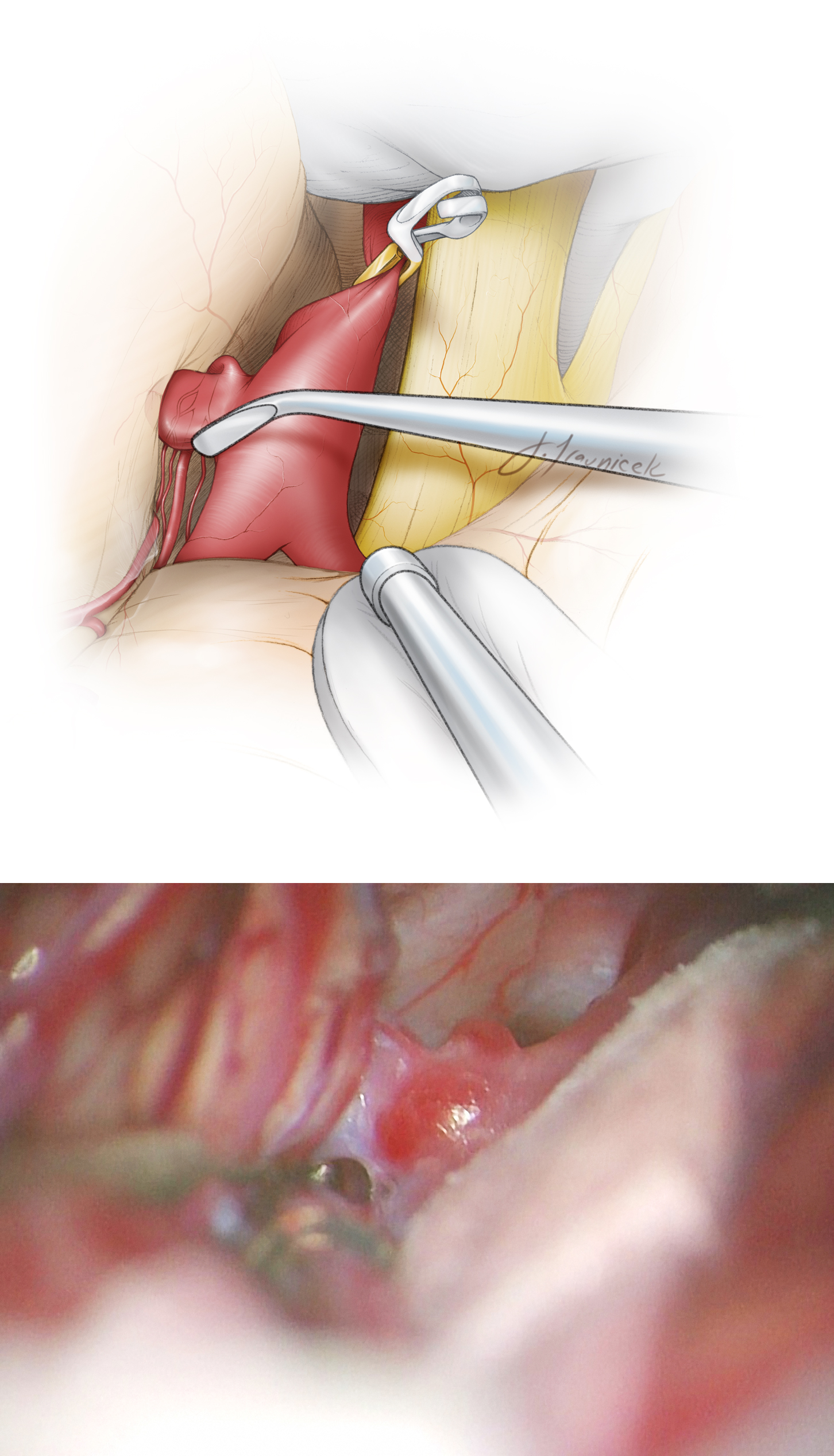
Figure 8: The aneurysm can be best manipulated and dissected under brief periods of temporary ICA occlusion. Clip application follows; the origin of the AChA should not be compromised. Invariably, a small neck remnant is necessary to preserve the AChA’s origin. Bayoneted clips are ideal for allowing me to visualize the origin of the AChA around the bulk of the clip appliers. The lower photo demonstrates one of the perforating branches of the AChA (arrow) just distal to the blades.
Figure 9: The clip was deployed and the AChA (red arrows) at the tip of the blades was inspected for its patency via intraoperative fluorescence angiogram.
As the clip blades are gradually closed, attention is focused on the tips of the blades to ensure that they are free of the AChA as it turns around the dome.
Figure 10: Once the clip is placed, I gently rotate the blades to ensure that the blades are not compromising any segment of the AChA. This inspection is crucial for avoiding complications; clip repositioning may be necessary. The arrow on the upper photo points at the origin of the AChA before clip deployment, while the arrow in the lower intraoperative photo points at the more distal route of the vessel around the aneurysm.
Fluorescence videoangiogram has some limitations for evaluation of AChA aneurysms after their ligation. Most often, because of the deep operative corridor and the hidden aneurysm, enough excitation light cannot reach the fluorescent agent within the sac for adequate emission signals. This phenomenon leads to false negative results. Therefore, puncture of the dome or intraoperative catheter angiogram is necessary for confirmation of aneurysm exclusion.
Additional Considerations and Aneurysm Variations
I prefer to use a simple bayonetted straight clip. If this is not anatomically feasible, as in the case of a posteriorly-projecting AChA aneurysms, I use an angled fenestrated clip, encasing the ICA. This construct avoids “dog ears” and is most effective for collapsing the neck. Furthermore, delayed clip displacement is unlikely.
Figure 11: I clip posteriorly-projecting AChA aneurysms using an angled fenestrated clip. The fenestration encircles the ICA. The AChA is seen just distal to the heel of the clip, proximal to the ICA bifurcation. This construct does not routinely require isolation of the AChA in case of its attachment to the dome.
Figure 12: The upper intraoperative photos demonstrate the configuration of a right-sided posteriorly projecting AChA aneurysm. The tip of the ball-tipped dissector points at the origin of the AChA (upper image). The angled fenestrated clip was effective while obviating the need for significant manipulation of the AChA (middle image). Intraoperative fluorescence angiography demonstrated patency of the AChA (arrow) and exclusion of the aneurysm.
Posteriorly Projecting AChA Aneurysm
Figure 13: An operative view of a fusiform AChA aneurysm without a definable neck is demonstrated. This aneurysm is not amenable to clip ligation. Wrapping the aneurysm with muslin is the most reasonable strategy in this case. The AChA should not be sacrificed.
Postoperative Considerations
Inadvertent sacrifice of the AChA leads to an anterior choroidal syndrome that consists of contralateral hemiplegia, hemianesthesia, and hemianopsia. This syndrome is very disabling and nearly always the result of trapping the AChA or perforators arising from the PCoA and AChA. Every effort should be made to avoid this unfortunate outcome.
Standard postoperative care is used. Anticonvulsants are recommended and tapered off about one week after surgery.
Pearls and Pitfalls
- The takeoff of the AChA can be mistaken for the proximal neck of the aneurysm. This anatomy must be carefully dissected to avoid occlusion of the AChA during aneurysm clipping.
- The proximal segment of the AChA typically drapes over the aneurysm sac. Careful microdissection is necessary to save the AChA during clip deployment.
References
Lawton M. Seven Aneurysms: Tenets and Techniques for Clipping. New York: Thieme Medical Publishers, 2011
Samson D, Batjer HH. Aneurysms of the posterior internal carotid wall, in: Intracranial Aneurysm Surgery: Techniques. Mount Kisco, NY: Futura Publishing Company, 1990.
Please login to post a comment.