Parietal and Occipital AVMs
This is a preview. Check to see if you have access to the full video. Check access
Parietal AVM: Managing Disturbing White matter Feeders
Please note the relevant information for patients suffering from arteriovenous malformations is presented in another chapter. Please click here for patient-related content.
Operative Anatomy
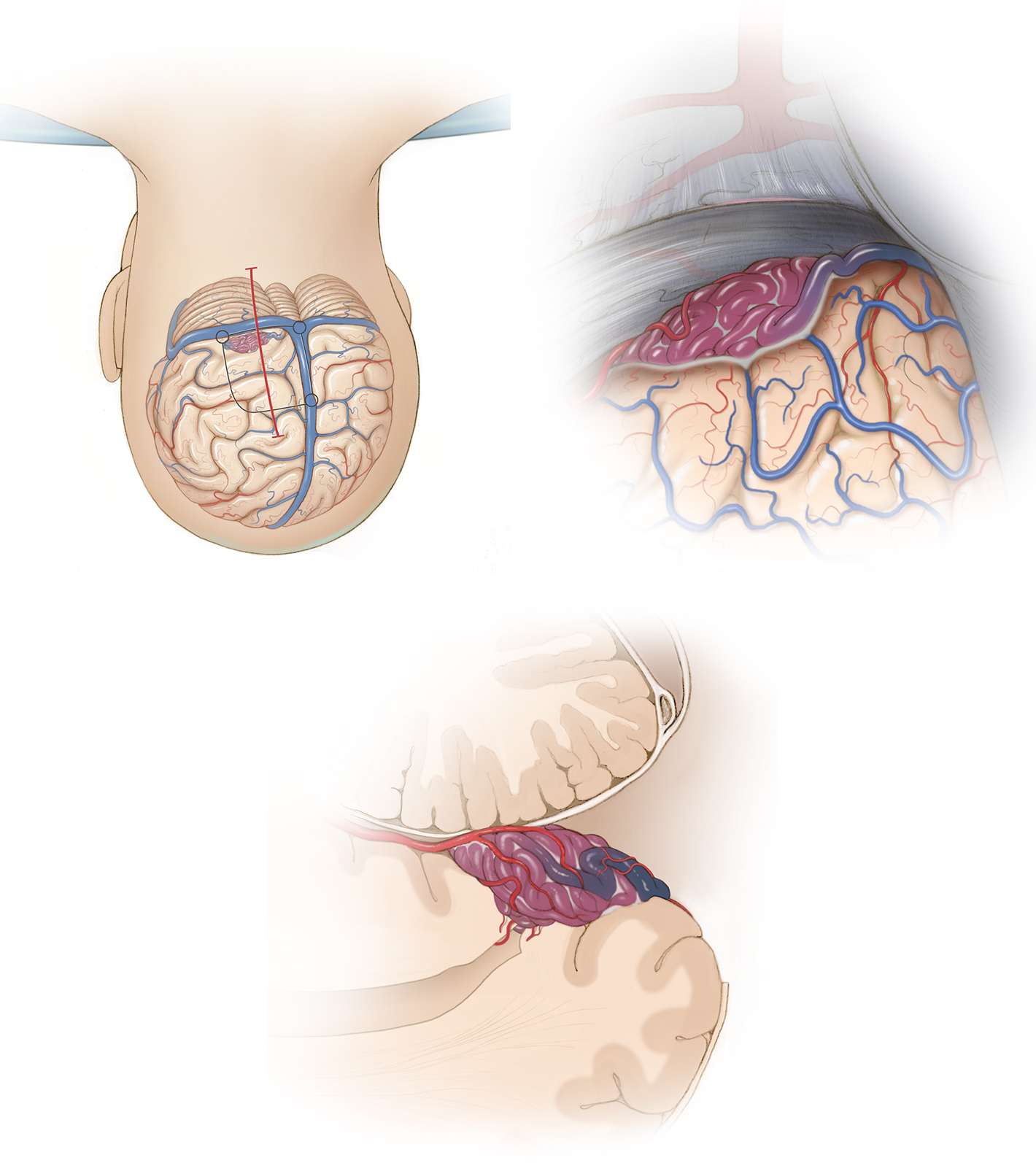
The parietal and the occipital lobes are neighbors with arbitrary boundaries. At their medial aspect, they are separated by the parieto-occipital sulcus. At their lateral surface, there is no real fissure or sulcus to demarcate them, but they may be separated arbitrarily by an imaginary extended Sylvian fissure line.
Because of such an intimate neighborhood, the vasculature of these two lobes is shared and interrelated. Therefore, arteriovenous malformations (AVMs) involve these lobes synchronously. As a result, I will consider these two lobes as a single unit during discussion of AVM excision.
All three major cerebral arteries and their branches supply the parietal and occipital lobes, including the middle cerebral artery (MCA), anterior cerebral artery (ACA), and posterior cerebral artery (PCA).
The distal and cortical branches of the MCA (both the superior and inferior trunks) supply the lateral parieto-occipital surface via:
- Central artery
- Anterior parietal artery
- Posterior parietal artery
- Angular artery
- Temporo-occipital artery
The distal ACA branches (A5) supply the medial parietal surface, including the cuneus and precuneus through the superior and inferior parietal arteries.
The major blood supply to the medial and basal parieto-occipital lobe is by means of the PCA and its branches:
- Posterior temporal artery
- Calcarine artery
- Parieto-occipital artery
These branches originate from the P3 segment of the PCA that forms near the posterior border of the midbrain and courses within the quadrigeminal cistern. On the other hand, the calcarine artery (the P4 segment of the PCA) courses through the calcarine fissure and supplies the inferior cuneus and lingula (the visual cortex).
The splenial artery separates proximally from the parieto-occipital artery at the posterior border of the splenium and anastomoses with the pericallosal artery, creating the “limbic loop.”
Click here to view the interactive module and related content for this image.
Figure 1: Surface anatomy of the lateral parietal and occipital lobes is shown (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 2: Surface anatomy of the medial parietal and occipital lobes is shown (images courtesy of AL Rhoton, Jr).
Venous drainage for the lateral surface primarily ascends to the superior sagittal sinus. The most dominant drainage system is the vein of Trolard. There are numerous anatomic variations, including double or triple Trolard veins. There is frequently no major draining vein along the distal 4- to 5-cm segment of the superior sagittal sinus except the lateral occipital vein.
Careful evaluation of the major parasagittal draining veins is a practical detail for feasibility of the posterior interhemispheric approach. Venous drainage is mostly en route to the Sylvian and occipital veins at the inferolateral surface of these lobes; these veins generally descend to the vein of Labbé.
Click here to view the interactive module and related content for this image.
Figure 3: Variations in the venous anatomy along the convexity are summarized. An absence of the vein of Labbé has led to an enlarged parasagittal vein along the posterior superior sagittal sinus (yellow arrow, right lower image) (images courtesy of AL Rhoton, Jr).
PARIETO-OCCIPITAL AVM SUBTYPES
Lateral Parieto-Occipital AVM
Lateral parieto-occipital AVMs are typical cone-shape convexity AVMs. These lesions may sometimes lack cortical representation and only manifest as an arterialized vein on the surface. This AVM subtype is considered eloquent when located on the postcentral gyrus or on the visual cortex at the medial occipital pole.
Feeding arteries are formed from the distal cortical MCA branches. However, ACA and PCA feeders may also participate at the depth of the AVM. Venous drainage is mostly superficial, ascends to the superior sagittal sinus and may also descend to the Sylvian vein or vein of Labbé.
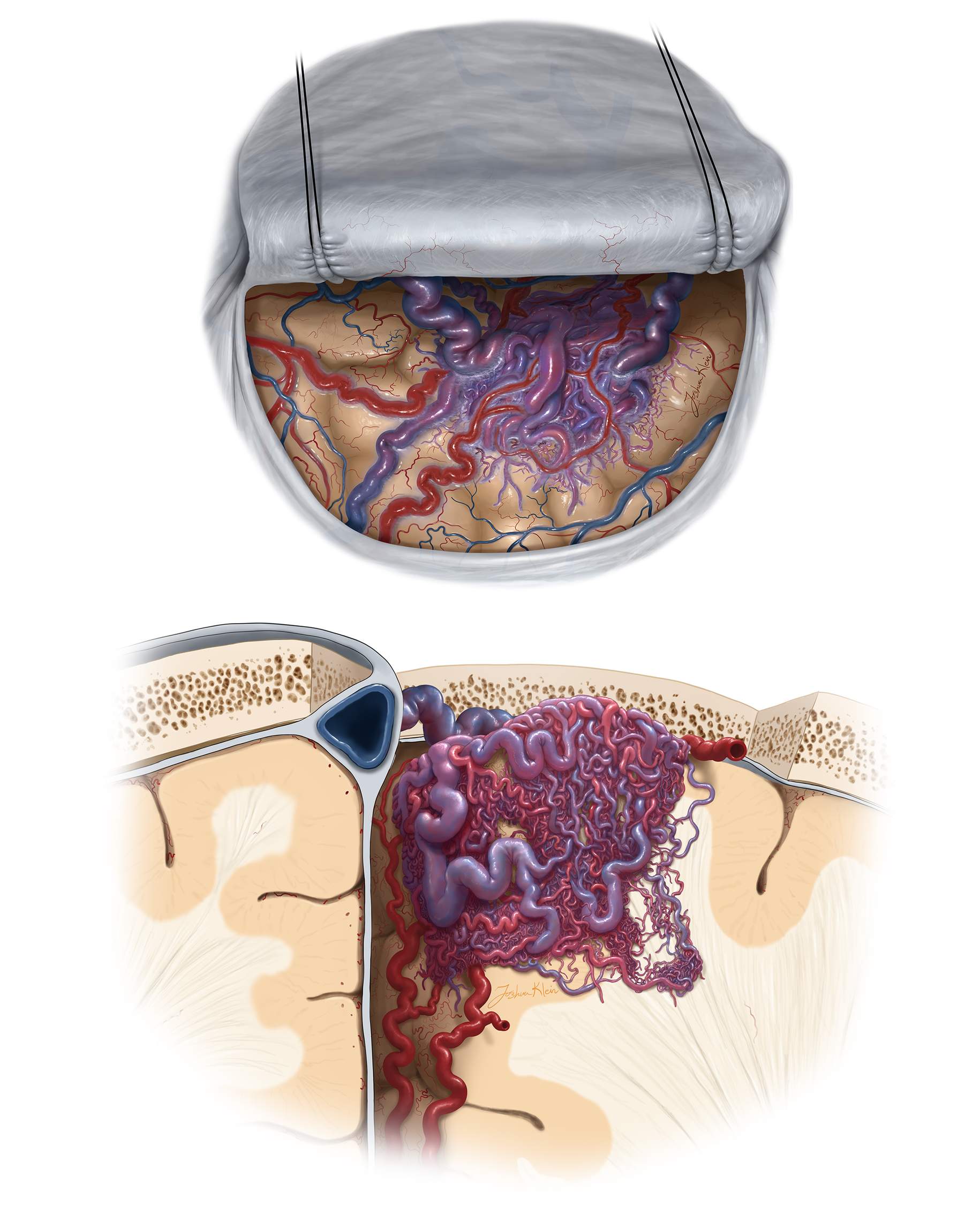
Figure 4: Lateral parieto-occipital AVM architecture is shown. Note the extension of larger nidi toward the ventricle. Involvement of the draining veins toward the superior sagittal sinus and sylvian fissure is also evident.
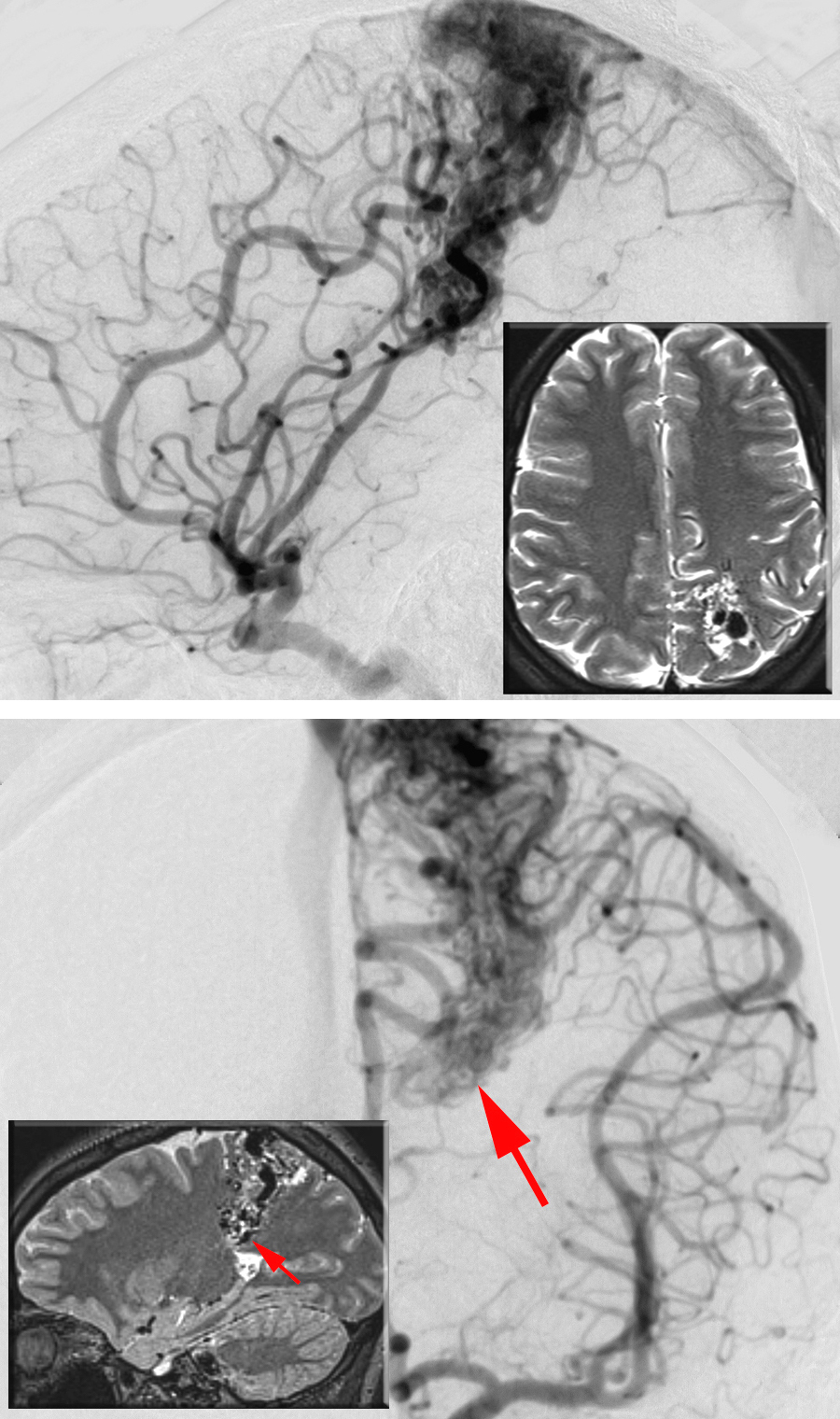
Figure 5: A lateral parietal AVM is shown on lateral (upper image) and anteroposterior (lower image) left-sided ICA angiograms. The inset MRIs illustrate the location of the AVM. The deep ependymal/choroidal feeding vessels near the ventricle (arrows) can be a challenging source of intraoperative bleeding.
Out-of-Control AVM: Getting out of Trouble
I prefer to position the patient in the lateral position; head rotation depends on the anteroposterior location of the lesion. The cortical presentation of the AVM is the highest point on the operative field. I use a U-shaped incision, encompassing the AVM and the surrounding normal brain margins. I incise the dura to circumscribe the lesion; the base of the dural flap is next to the venous sinus.
Dissection begins at the margins of the AVM by opening the thick arachnoid layers and sulci to identify, follow, and then divide the feeders near the nidus. The primary feeders are mostly recruited from the distal cortical branches of the MCA that are found at the anteroinferior margin of the AVM. Therefore, this area is disconnected first.
Large AVMs reach into the occipital horn or atrium and engage feeders from the choroidal/ependymal arteries or deep MCA branches. Parenchymal dissection is conducted by “hugging” the nidus with great delicacy, especially at the eloquent sides of the AVM, protecting the central lobule, Wernicke’s area, and the visual cortex as well as their radiating fiber tracts. The deep white matter feeders in this area can be unsettling to the surgeon; their aggressive pursuit can lead to injury to the descending fibers of the central lobule.
All venous drainage to the vein of Labbé, superficial temporal veins, and superior sagittal sinus should be saved intact until the completion of circumdissection of the nidus. The lobules of the AVM projecting into the white matter should not be inadvertently transected and left behind. This inadvertent error can especially occur at the level of the periventricle. The residual nidus is the source of intraoperative bleeding and unexpected brain swelling, notably if the bleeding fills the ventricles.
AVMs located on the inferior edge of the central sulcus should be treated like Sylvian frontal AVMs and approached with the same surgical considerations, including careful Sylvian fissure dissection and preservation of en passage or bystander arteries.
Medial Parieto-Occipital AVMs
Medial parieto-occipital AVMs are located on the medial aspect of the hemisphere without any extension or presentation on the lateral convexity. They usually do not involve the splenium of the corpus callosum.
The location of the lesion with respect to the parieto-occipital sulcus defines a medial parietal or occipital AVM; these AVMs share a similar surgical approach and technical nuances. Their main feeders arise from the PCA branches, including the parieto-occipital or Calcarine artery and distal ACA branches, including the paracentral artery, as well as the superior and inferior parietal arteries. Complex nidi also procure feeders from the distal MCA branches.
The dominant drainage system is cortical, ascending to the superior sagittal sinus and/or descending to the inferior sagittal sinus and then leading to the straight sinus and finally into the vein of Galen.
Figure 6: The angioarchitecture of this AVM subtype is demonstrated. Note the above described feeding arteries and draining veins. Larger AVMs reach the atrium and receive choroidal deep white matter feeders.
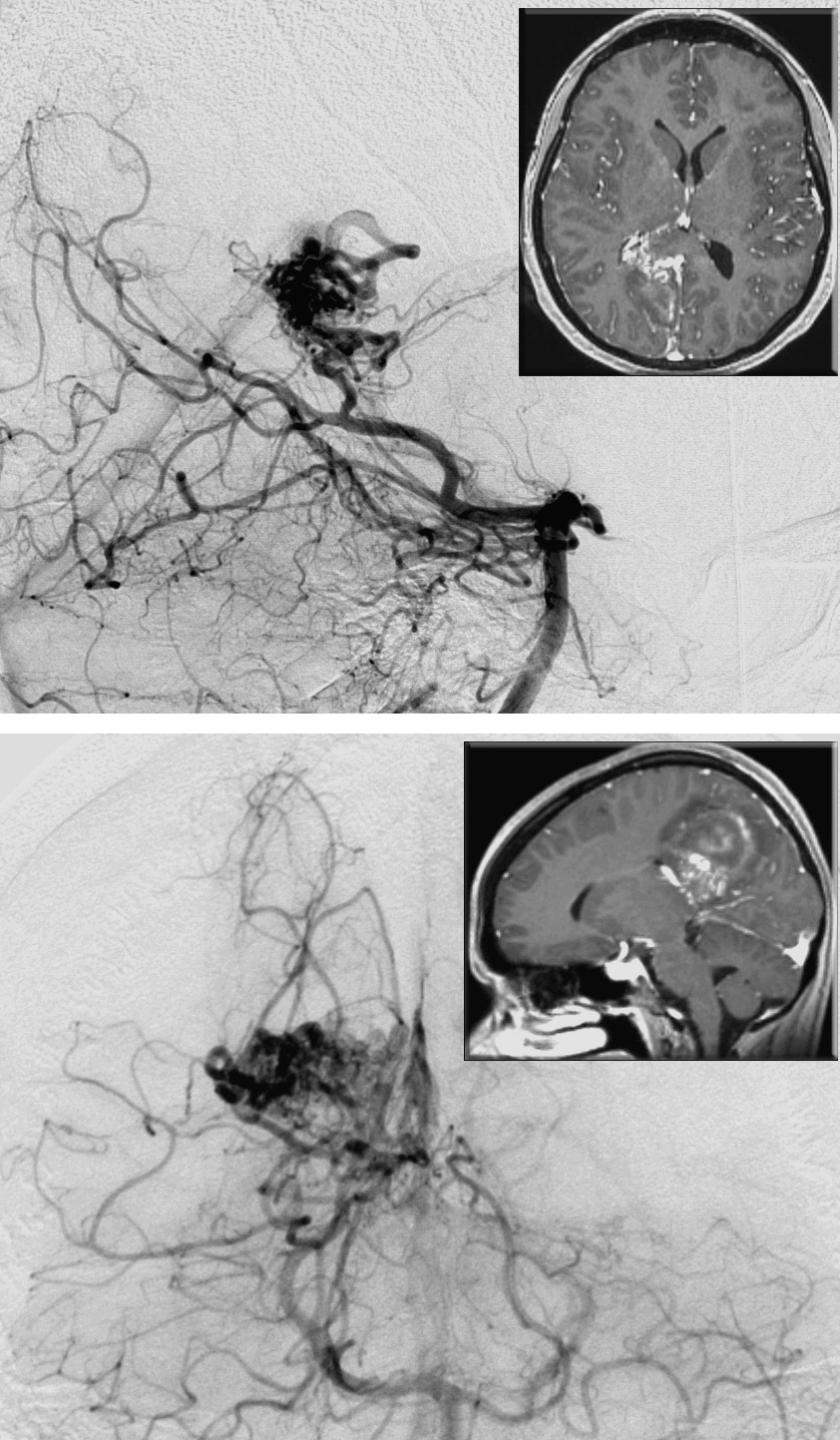
Figure 7: This hemorrhagic medial parietal AVM is primarily fed by the branches of the distal PCA. Note the lateral (upper image) and anteroposterior (lower image) vertebral angiograms and corresponding MRIs (insets). The nidus reaches the atrium. The ipsilateral interhemispheric approach may not safely reach the periventricular extent of the lesion.
Medial Parietal and Peri-atrial AVM: Transfalcine Approach
The small and superficial medial parietal lesions can be reached via the ipsilateral interhemispheric corridor; I use the contralateral interhemispheric transfalcine approach for lesions with predominant subcortical extension to avoid aggressive lobar retraction.
Regardless of the operative route, the surgeon’s view is tangential to the surface of the AVM without any obvious convexity cortical access. Since the operative trajectory is oblique, I use the lateral head position with the AVM in the dependent hemisphere for the ipsilateral route and the unaffected hemisphere in the dependent position for the contralateral transfalcine approach.
For medial parietal AVMs, the position of the patient’s head is midline horizontal (the axis of the sagittal suture is parallel to the floor), but for medial occipital AVMs, I turn the head 45 degrees toward the floor (“nose down”) to allow the occipital lobe to fall away from the falx and open the operative working corridor. I install a lumbar drain to release cerebrospinal fluid (CSF) early and facilitate interhemispheric dissection.
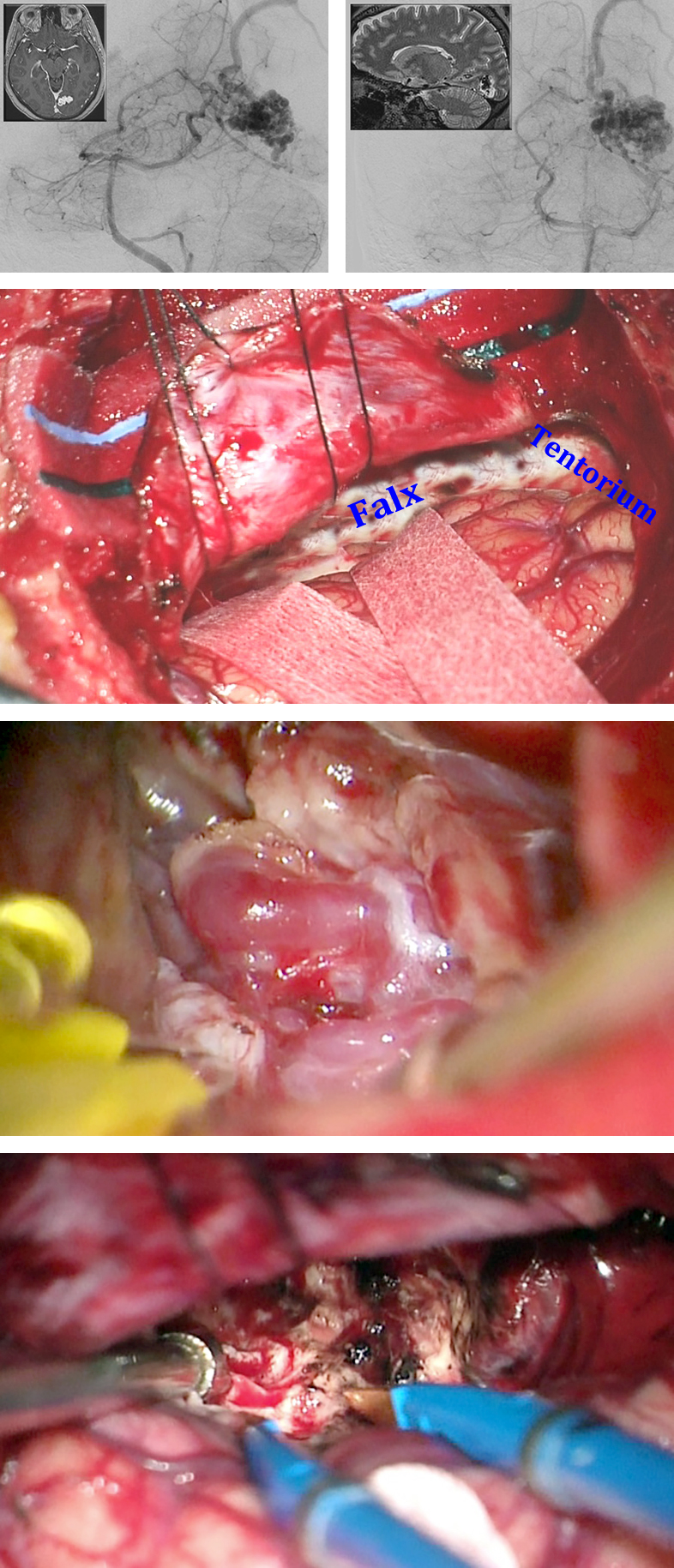
The incision is semicircular or U-shaped with a wide vascular pedicle. The craniotomy should be generous and for occipital AVMs should expose the posterior superior sagittal sinus, a portion of the torcula, and the ipsilateral transverse sinus. The base of the dural opening is divided on the superior sagittal sinus medially and the transverse sinus inferiorly. I dissect along the falx within the interhemispheric fissure to reach the falcotentorial junction, and open the quadrigeminal cistern to drain additional CSF and slack the brain.
Parasagittal draining veins can impede arachnoid dissection by tethering the medial hemisphere to the midline and counteracting gravity retraction. The veins can be carefully untethered along their subdural length over the interhemispheric space so that the hemisphere can be mobilized. However, they should be saved until the end of nidal dissection. This anatomic obstacle should be noted preoperatively and a plan formulated for a wider and adjusted craniotomy so that the appropriate working angles around the veins can be attained.
The steps for AVM devascularization are as follows: First, I identify the interhemispheric ACA feeders, isolate them to the level of the nidus, and disconnect them. Distal ACA feeders are typically found at the anterior and inferior margins of the AVM. These feeders are mostly terminal ACA branches that can be safely sacrificed. Next, I isolate the PCA feeders at the posterior border of the AVM; these vessels can be en passage arteries and therefore require skeletonization.
I continue pial and sulcal dissection along the superior and posterior margins and progress in a spiral-like fashion circumferentially around the deeper sections of the lesion.
After superior and posterior dissection, the AVM can be mobilized medially into the interhemispheric fissure. This maneuver allows me to reach and work on the anterior and inferior fronts of the lesion for disconnection of additional PCA feeders. The white matter feeders can be problematic as the periventricular dissection is in progress. In select cases, resection should be extended to the level of the ependyma to ensure total nidal removal and obliteration of all arteriovenous shunting.
For larger AVM that reach the pri-trigone region, I favor the contralateral interhemispheric transfalcine approach. This oblique operative trajectory is founded on the concept of using a contralateral operative route to augment a more tangential working angle toward the more difficult-to-reach lateral target through a midline reach. This approach provides a more expanded access to the midline and deep feeders of the AVM as compared to the ipsilateral pathway.
Figure 8: The contralateral interhemispheric transfalcine approach was used for medial parietal AVMs with extension toward the trigone. Note the transfalcine incisions to exposure the AVM in the top two images. The retention sutures in the falx and the falcine flaps maintained the exposure to the medial hemisphere. The draining veins and feeding arteries were readily exposed and nidus removal unveiled the atrium (lower two rows).
Medial Occipital AVM
Paramedian Parieto-Occipital AVMs
Paramedian parieto-occipital AVMs are a composite of lateral and medial parieto-occipital AVMs. Accordingly, depending on their size and complexity, they may procure feeders from all three major cerebral vessels: MCA, ACA, and PCA. Parieto-occipital AVMs are eloquent if they involve the paracentral lobule or the occipital pole. Venous drainage mostly ascends and drains into the superior sagittal sinus.
Similar to paramedian frontal AVMs, I work on the medial and lateral surfaces to start nidal isolation. Unfortunately, engorged and fragile parasagittal draining veins tether the medial hemisphere to the midline, negating gravity-dependent retraction. These veins are prone to premature rupture and should be carefully handled.
Figure 9: The angioarchitecture of this AVM subtype is demonstrated. Note the above described feeding arteries and draining veins. The more superficial location of these lesions makes their resection less technically challenging.
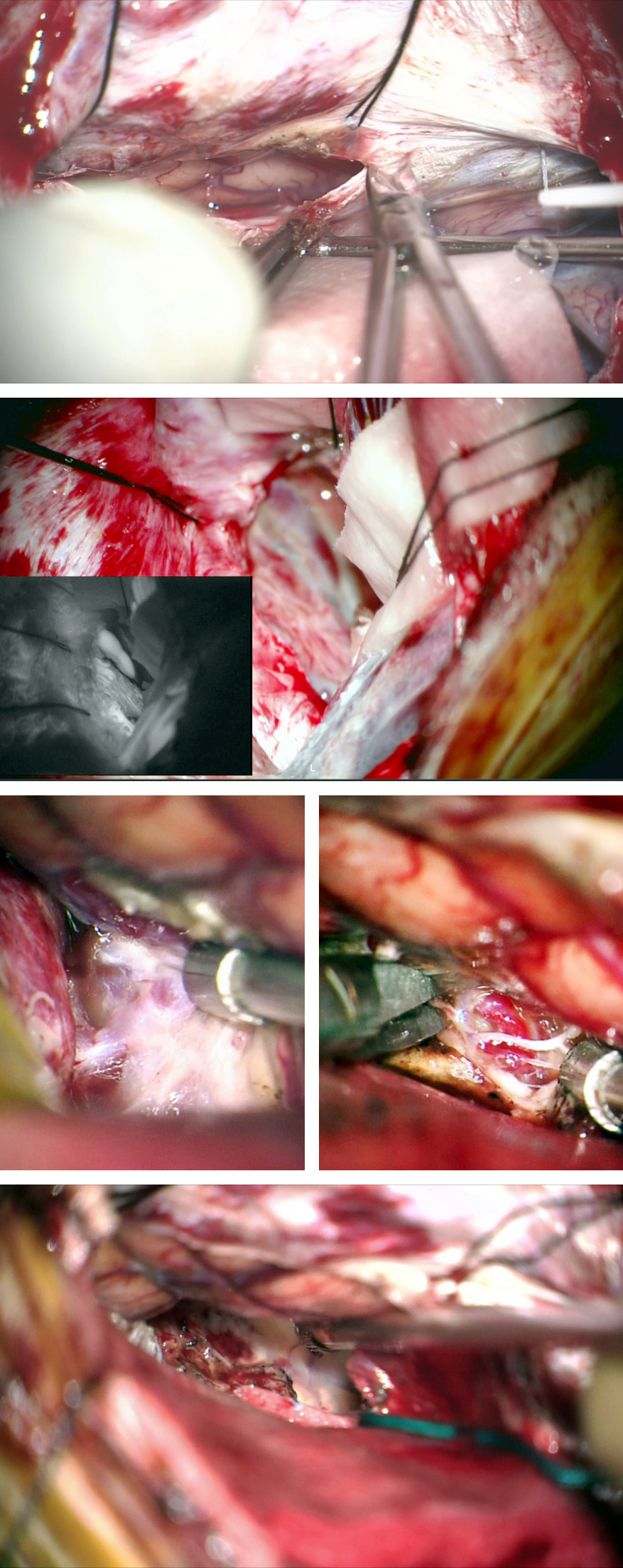
Figure 10: A paramedian parieto-occipital AVM is shown (upper images-lateral and anteroposterior vertebral angiograms). This lesion is primarily fed by the PCA. The preoperative overembolization of the larger feeding pedicles potentially led to the intraoperative findings of white matter feeder hypertrophy and excessive blood loss during disconnection of the white matter feeders (lower photo).
For paramedian anterior parietal AVMs, I position the patient’s body and head in the supine position (“nose up”) without significant rotation; the neck is flexed. This configuration allows me to work on the lateral cortical aspect of the AVM, but gravity retraction is no longer available for the medial aspect. Since the patient’s head is substantially above the level of the heart, appropriate measures for detection and management of venous air embolism are appropriate.
Figure 11: For paramedian occipital AVMs, I prefer the lateral body position with contralateral head rotation so that the midline is almost vertical, leaving the AVM at about the highest point in the operative field. More table rotation will place the midline exactly vertical, allowing access to the interhemispheric space. Tilting the bed 45 degrees will place the midline along an oblique plane while I work on the lateral convexity. A paramedian linear incision is demonstrated; a horseshoe incision is an alternative option.
Occipital AVM: Controlling the Bleeding
A generous parietal craniotomy exposes the midline and superior sagittal sinus for paramedian parietal AVMs. Similarly, a generous parieto-occipital craniotomy is used for paramedian occipital AVMs, exposing the torcula, superior sagittal sinus, and transverse sinus. A wide dural opening based medially on the superior sagittal sinus and inferiorly on the transverse sinus for an occipital craniotomy is necessary.
It is important to appreciate the normal cortical draining veins that can interdigitate prematurely with the parasagittal arterialized veins of the AVM before reaching the superior sagittal sinus. It is ideal to protect the normal veins during the dural opening (leaving a sleeve of dura around them) and during the sacrifice of the associated arterialized veins at the end of the nidal resection.
The AVM disconnection is performed in a stepwise fashion. First, I dissect along the interhemispheric fissure to carefully untether the draining veins and expand the corresponding operative corridor. Next, the feeding arteries, usually the distal ACA feeders, are disconnected precisely at their terminal entry point into the anterior margin of the malformation.
The lateral sulci at the AVM margin over the convexity are then dissected and the MCA feeders are severed. The sensorimotor cortex and its en passage vessels are protected along the anterior border. Circumferential dissection progresses to mobilize the AVM out of the resection cavity after its deep disconnection from the MCA feeding arteries and ependymal contributories near the occipital horn or trigone.
Medial mobilization of the lesion into the interhemispheric corridor allows me to divide the feeders from the PCA at the inferior and lateral margins. During these maneuvers, I tilt and rotate the bed to achieve appropriate working angles.
Basal Occipital AVMs
Basal occipital AVMs are located on the basal surface of the occipital lobe and are the least common of the parieto-occipital AVMs. Feeding vessels originate from the PCA via the posterior temporal and calcarine branches. Distal MCA branches (including temporo-occipital artery) may sometimes be involved as well.
Figure 12: The prone patient positioning (top row left) and the operative exposure of the AVM is noted (top row right and bottom row.) The last image is a sagittal view of the malformation in relationship to the tentorium and occipital horn of the lateral ventricle.
Figure 13: A medial basal occipital AVM is evident on the oblique and anteroposterior vertebral angiograms (top images). The draining vein travelled superiorly. Although an infraoccipital trajectory would be reasonable, I approached this lesion through a combined occipital interhemispheric and infraoccipital route (second row). The lower photos show the nidus and the resection cavity.
Basal Occipital AVMs
Venous drainage is typically superficial via the temporobasal and occipitobasal veins joining the transverse or the tentorial sinuses. Basal occipital AVMs are approached via a supratentorial-infraoccipital trajectory. I prefer the lateral body position while the patient’s head is turned toward the floor, placing the midline almost vertical. Using neck flexion, I try to exploit gravity retraction on the occipital pole. CSF release via the lumbar drain is necessary for smooth elevation of the occipital lobe during the early stages of the operation, before the cisterns are reached.
For lesions located medially on the basal occipital lobe, the torcula is unroofed. A standard occipital craniotomy is appropriate for exposure of the AVMs located laterally on the inferior occipital gyrus; the transverse sinus is unroofed.
The dural opening is based on the transverse sinus. Next, I reach over the tentorial incisura to open the quadrigeminal cistern and drain additional volume of CSF to slacken the brain. Sulcal dissection starts at the posterior AVM’s margin and extends to the medial and lateral borders sequentially. Deep dissection mobilizes/drops the nidus inferiorly, toward the tentorium, so that I can expose and manage the anterior feeders from the posterior temporal and calcarine arteries.
“Tight” dissection planes, “hugging” the nidus, are mandatory to minimize the risk of visual field deficits. The deep white matter feeders along the superior pole of the nidus (within the operative blind spot) can be problematic.
Contributors: Rouzbeh Shams-Amiri, MD, and Mohsen Noori, MD
References
Lawton MT. Seven AVMs: Tenets and Techniques for Resection. New York, Stuttgart: Thieme Medical Publishers, 2014.
Please login to post a comment.