Acute Subdural Hematoma
This is a preview. Check to see if you have access to the full video. Check access
Evacuation of an Acute Subdural Hematoma
In contrast with a treatable epidural hematoma (EDH), an acute subdural hematoma (ASDH) carries a high risk of morbidity and mortality, even with timely decompression.
The difference in outcome between EDH and ASDH arises from two discrete but related etiologies. First, the force of trauma or impact necessary to cause an ASDH is significantly greater than that required for an EDH. The typical scenario for an ASDH involves an acceleration-deceleration shear injury (e.g., a motor vehicle accident, fall, or whiplash injury) during which the brain’s inertia continues to move the brain after the body and skull have stopped moving. This phenomenon results in significant shear vectors affecting the brain parenchyma and its tethered and vulnerable venous system. Diffuse axonal injury is common among patients suffering from ASDH.
Second, with ASDH, there is an increased likelihood of injury to the underlying brain parenchyma. Thus, the ASDH has two potential sources of hematoma formation: the brain parenchyma and the parasagittal bridging veins. A laceration of the brain resulting in an expanding hematoma is the more ominous of the two because it implies significant parenchymal injury.
A distinction must be made between a chronic subdural hematoma and an acute subdural hematoma. An ASDH is defined as a bleed occurring within the subdural space that occurred within 72 hours prior to the patient’s presentation. Bleeds in patients who present after 72 hours of injury are considered either subacute or chronic, depending on their timing and the computed tomography (CT) findings. The mechanisms and rates of hematoma expansion for acute and chronic SDHs and their corresponding outcomes are different. This chapter primarily deals with ASDHs.
Presentation
An ASDH occurs after a traumatic event involving acceleration-deceleration forces, rather than the blunt trauma and skull fracture that are typically associated with EDH. Due to the impact of trauma, the patient is often in a comatose state with a Glasgow coma scale (GCS) score ≤8, necessitating the dispatch of emergency personnel and rapid transport of the patient to a trauma center.
In contrast with EDH, there is often no lucid interval for a patient with ASDH. The signs and symptoms of transtentorial or uncal herniation may be present and are dependent on the extent of the enlarging hematoma. However, the absence of these findings does not necessarily imply a good prognosis because brain parenchymal contusion and/or laceration can still lead to significant morbidity and mortality.
Some patients may remain conscious after their injury, but their condition subsequently deteriorates as the hematoma enlarges with or without signs of herniation. For patients who remain conscious after an accident with an associated high propensity for ASDH formation, it is important that the physician maintains a high index of suspicion for hematoma expansion and neurologic deterioration.
Diagnosis
As is the case for all patients with a potential traumatic brain injury (TBI), it is imperative to obtain a head CT scan without contrast in order to adequately detect an expanding hematoma. On noncontrast CT, an expanding ASDH will appear as an intensely white, crescent-shaped mass situated between the brain parenchyma and the inner table of the skull. ASDH is most commonly unilateral, but some patients may harbor bilateral ASDHs.
An ASDH, by definition, is located between the dura mater and subarachnoid mater; thus, the expanding hematoma is not limited by the sutures of the calvarium, but rather by the dural sinuses. This property allows the ASDH to outline the entire brain hemisphere. It may be helpful to identify the volume of blood but the amount of midline shift is the key determinant for the need to proceed with surgical decompression. A small hematoma may require evaluation if the underlying brain edema is exacerbating the midline shift, leading to a depressed level of consciousness; this is not an uncommon phenomena among pediatric patients suffering from ASDH.
Finally, the frontal and temporal lobes should be examined to exclude concurrent parenchymal injuries or contusions.
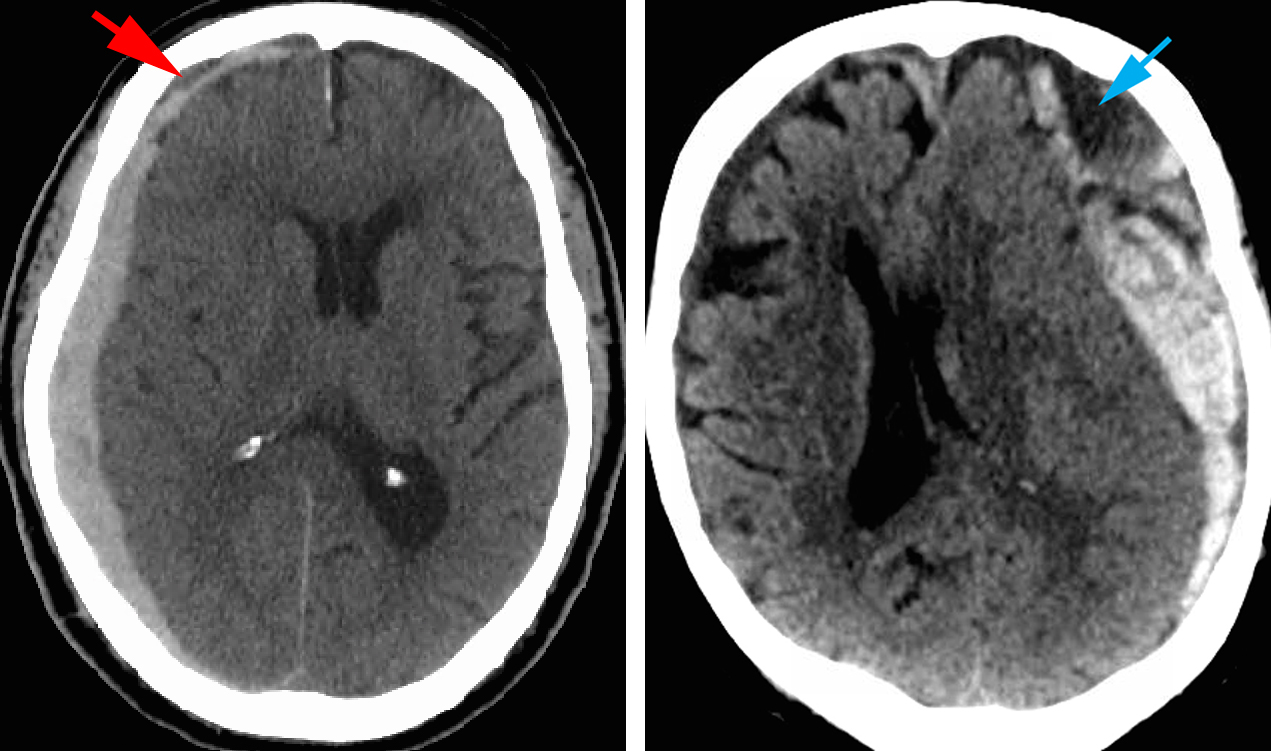
Figure 1: CT scans of subdural hematomas: The left image demonstrates a right-sided acute subdural hematoma with evidence of small hyperacute component (hypodense, red arrow). The right image demonstrates a left-sided acute subdural hemorrhage in the presence of a pre-existing chronic subdural hematoma (blue arrow). As demonstrated by these scans, the ages of the patients are very different.
Skull fractures around the dural venous sinuses should be carefully studied.
Indications for Surgery
The following guidelines are not definitive, but should be considered in the decision-making process for surgical management of ASDH:
- ASDHs with thickness >10 mm or midline shift >5 mm on CT scan
- ASDHs with a thickness <10 mm and midline shift <5 mm on CT may need to be surgically treated under the following conditions:
- GCS drops by ≥2 between injury and admission.
- Pupils are asymmetric or fixed and dilated.
- Intracranial pressure is >20 mmHg.
- All patients with GCS <9 should have intracranial pressure (ICP) monitoring implants.
Although these guidelines provide a framework for operative decision-making, it is important to consider the overall clinical picture and exam in assessment of the patient. Specifically, a poor GCS score (GCS <5), old age, and massive brain herniation (as determined by pupils’ status) predict a high mortality rate regardless of surgical intervention and medical management. Thus, even when all of the operative guidelines are met, there are scenarios that call for operative restraint and palliative management. Concurrent multifocal intracerebral contusions are associated with poor prognosis and should be considered during selection of the treatment strategy.
Conservative management may be used when the operative guidelines are not met, but this decision does not rule out the potential need for operative management in the near future. Moreover, conservative management indicates frequent neurologic evaluations, potential need for ICP monitoring, and serial CT scans for continual assessment of hematoma status, evolution of contusions, and brain edema.
Although the initial CT scan may not be very concerning in the case of a comatose patient, initial ICP measurements after implantation of the monitor may indicate a need for an emergent operative intervention and a decompressive craniectomy.
Preoperative Considerations
Additional diagnostic studies are necessary at presentation. The patient’s prothrombin time (PT), partial thromboplastin time (PTT), and international normalized ratio (INR) are assessed. The use of antiplatelet and anticoagulation medications should be reviewed. Correction of coagulation and platelet dysfunctions prior to surgery should be considered. In the absence of other coagulation abnormalities, I do not reverse the effect of acetylsalicylic acid (ASA) derivatives (aspirin).
The patient’s presenting neurologic status may be concerning or may continue to deteriorate after admission to the hospital. In almost any instance, it is preferential to obtain a head CT before proceeding with surgical decompression. If increasing intracranial pressure is responsible for the patient’s neurologic deterioration, appropriate measures should be instituted, including hyperventilation (PCO2 of 30-35 mmHg), administration of mannitol, and head elevation.
The patient must not be overly hyperventilated because this may lead to cerebral ischemia secondary to the excessive cerebral vasoconstriction and coexisting pressure gradients. Immediate neurosurgical evaluation and triage is crucial.
SURGICAL EVACUATION OF ACUTE SUBDURAL HEMATOMA
Once a decision is made to proceed with surgical decompression, the procedure should be performed as soon as possible if symptomatic mass effect is present. Two variables have been shown to correlate with outcome: 1) timing of the operation, and 2) strict control of the patient’s ICP. Numerous studies have supported the theory that the best operative outcomes for patients with ASDH occur when the procedure takes place within 4 hours of the injury.
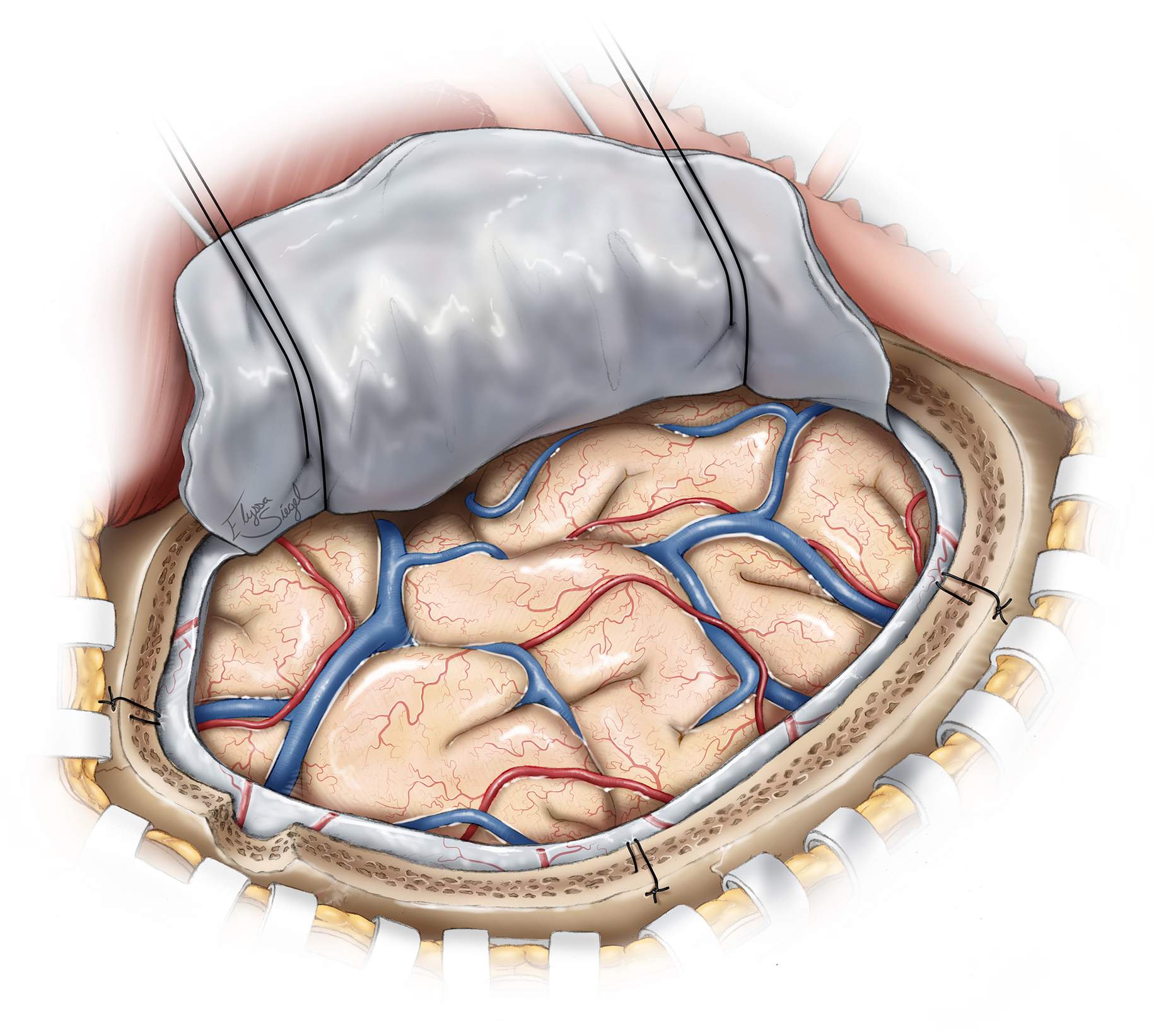
Figure 2: The patient is placed in the supine position with the head resting on a donut gel pad. A large horseshoe incision “trauma flap” is outlined (green line). The red line outlines the incision for a smaller ASDH with mainly frontal extension. The incision starts at the level of zygomatic arch, 1 cm anterior to the ear, and proceeds superiorly and then posteriorly around the helix of the ear; the parietal bone is included within the incision outline. Once the incision approaches the calvarial midline, it is brought anteriorly to the level of the forehead just behind the hairline. This large scalp flap allows for a generous craniectomy if cerebral swelling occurs upon hematoma evacuation.
Following completion of the incision, I reflect a myocutaneous flap anteriorly. An expanded burr hole may be placed in the temporal bone and the dura incised in a cruciate fashion to allow the hematoma to extrude out through the burr hole. This maneuver may be used for rapidly deteriorating patients.
Figure 3: The location of the burr hole and the outlines of the bone flaps are shown. The typical bone flap (black dashed line) provides access to most of the hemisphere. The smaller bone flap (red dashed line) exposes the ASDHs affecting the anterior hemisphere. The bone flap must provide access to the anterior and middle cranial fossae due to the propensity of contusions to occur within these lobes. Additionally, the superior portion of the bone flap should extend to within ~2 to 3 cm of the sagittal suture; this consideration allows adequate visualization of the parasagittal bridging veins (often the source of hemorrhage).
I believe it is usually possible to remove most convexity ASDHs (regardless of their size) through the smaller bone flap (red dashed line). During the later stages of the procedure (please see below), I carefully work beyond the edges of the craniotomy along the subdural space to evacuate any additional hematoma compartments not directly exposed. If a generous craniectomy is indicated in the face of malignant cerebral swelling, the scalp should heave been prepared so that a “T” incision can be made along the mid portion of the incision for posterior extension of the craniotomy.
Figure 4: The myocutaneous flap is mobilized. The burr hole is placed in the temporal bone and can provide a quick method of decompression if the dura is incised. The outline of the craniotomy is illustrated.
The methods of incising the dura for an ASDH should be well thought out because the underlying brain injury may have resulted in significant brain edema, which can lead to massive brain herniation upon opening the dura.
Moreover, the edema may leave very little space between the dura and brain along the edges of the hematoma, thus causing cortical disruption when the dura is opened with scissors. The dural incision should typically follow the outline of the bone flap while leaving enough dura at the edge of the flap to allow for dural closure after evacuation of the hematoma.
If significant brain edema is present (or the extent of mass effect is out of proportion to the size of the hematoma) on the preoperative scans, numerous dural linear incisions or slits (~3 to 4 cm in length) may be made and the hematoma evacuated while the operator works between these slits. This maneuver will prevent massive cerebral herniation after a wide dural opening, which could lead to hemodynamic instability because of acute cerebral and brainstem dislocations.
Figure 5: Incising the dura. As shown, the dura is elevated with small forceps, and scissors are used to complete the dural incision. The incision follows the contours of the bone flap with enough dura left at the edges to allow for approximation of the dural edges during closure.
INTRADURAL PROCEDURE
Rapid decompression of the hematoma is the most effective method to relieve intracranial tension.
Figure 6: Once the dura is properly tacked up, the hematoma is mobilized using a combination of a #3 Penfield dissector, gentle suction, and irrigation fluid (hydrodissection). The dissector and gentle force of the irrigant elevate the hematoma from the cortex while suction removes this portion of the hematoma. This maneuver prevents the direct effect of suction on the edematous cortex and its friable vessels. The hematoma located beyond the edges of the craniotomy can be removed using the gentle hydrodissection effect of irrigation fluid. The cortex is gently held away from the edges of the craniotomy using a small piece of cottonoid patty while the hematoma fragments are “irrigated out” (inset image). Blind use of suction beyond the edges of the craniotomy is prohibited because this could lead to additional injury to the bridging veins as well as occult bleeding that could be difficult to reach and control.
Any obvious small cortical bleeding veins or arteries should be controlled with bipolar electrocautery. The subdural space beyond the edges of the craniotomy should be carefully re-explored, and additional remote clots removed as described above. I attempt to remove most mobile hematoma fragments that do not reside close to the dural venous sinuses.
Smaller clots that are not well visualized or reside near the dural sinuses or other large vital bridging/cortical veins (such as the vein of Labbe) should be left alone and undisturbed. Manipulation of these clots can uncover the original source of the hematoma; these venous channels are difficult to control without their sacrifice, and they invariably lead to more bleeding if they are manipulated. Aggressive coagulation and packing of hemostatic materials will further compromise the lumen of the venous drainage systems and lead to exacerbation of cerebral swelling.
The subdural spaces along the middle and anterior cranial fossae must also be inspected to ensure adequate hematoma evacuation. This tactic will potentially help with compromised cerebrospinal fluid flow and resolve uncal herniation.
Figure 7: Next, the visible cortex should be inspected for obvious contusions and necrotic areas. Necrotic areas are evident because of their bluish discoloration. If significant brain tension is present despite the removal of the hematoma, the large contusions and necrotic areas may be aspirated. This maneuver is not without risk as it may lead to additional bleeding that may be difficult to control because of an edematous and heavily injured brain in patients who have suffered from ASDHs.
Complication Management
During ASDH evacuation, brain edema is most likely related to the loss of cerebrovascular autoregulation and reactivity. The intense cerebral tension may be startling to the surgeon because it can lead to massive brain herniation through the bony defect. This phenomenon results in cerebral and brainstem dislocation and associated hemodynamic instability. It can also impede proper dural closure at the completion of the operation.
It is imperative to rapidly attempt to tamper the amount of edema with the following interventions. First, the surgeon and anesthesiologist should ensure that the patient is properly positioned, the jugular venous drainage is not compromised, and the endotracheal tube is not kinked. Next, the anesthesiologist should induce the patient into a state of mild arterial hypotension while avoiding any significant risk of ischemia. As mentioned above, the cerebral vascular autoregulation is markedly impaired; this mechanism is one of the common reasons for massive intraoperative brain swelling during ASDH surgery.
A barbiturate coma may be induced while the surgeon completes a temporal or frontal lobectomy as a final decisive step. If difficult or refractory swelling is encountered, the operator must consider the potential presence of a contralateral epidural, subdural, and/or intracerebral hematoma(s) as causative factors. These pathologies should be ruled out using intraoperative Doppler ultrasonography or a postoperative CT scan. Ultimately, rapid dural and scalp closure without replacement of the bone flap is prudent.
Intraoperative bleeding or venous “ooze” is common during removal of ASDHs. Undetectable irregularities in coagulation cascades are often present after massive brain injury. As ASDHs are most commonly caused by shear injury of the parasagittal veins, recurrent intraoperative bleeding is also usually venous in origin.
For more information regarding management of intraoperative cerebral swelling, refer to the chapter on Brain Swelling.
Closure
If the operator has any suspicion of brain swelling after hematoma evacuation, the dura is loosely approximated, a duraplasty performed using a piece of pericranium and the bone flap not replaced. Upon closure of the dura, tack-up sutures are placed at the edge of the craniotomy and, if a potential space is present, in the middle of the dural flap.
Finally, many patients who undergo the operation will need ICP monitoring postoperatively; placement of an intraparenchymal monitor is reasonable. A contralateral ventriculostomy may also be considered if significant ICP issues are expected postoperatively.
Postoperative Considerations
Strict control of the ICP is crucial for minimizing secondary brain injury postoperatively. The rise in ICP occurs most often because of a defective autoregulatory mechanism of the cerebral blood flow. A CT scan should be performed on the first postoperative day to assess for any residual, recurrent or distant hematomas. Serial CT scans should also be obtained in a patient who suffered from an unexplained rise in ICP or who fails to improve neurologically.
Patients with TBI commonly experience seizures before and after surgical intervention. The patient should be loaded with a preferred anticonvulsant drug either prior to or immediately after surgery to prevent the occurrence of early seizures. Early seizures are defined as those occurring within one week of the insult, and they do not necessarily imply epilepsy or the need for life-long anticonvulsant therapy. Unexplained lack of neurological improvement or deterioration may indicate subclinical seizures and electroencephalographic monitoring is recommended.
Finally, extracranial complications are common in patients with acute head injuries. Pulmonary sequelae, including acute respiratory failure or infections, should be closely monitored and pre-emptively treated. The increased ICP may also lead to Cushing ulcers and should be prophylactically treated through the administration of proton pump inhibitors or histamine blockers.
Pearls and Pitfalls
- The mechanisms of brain trauma are different in EDHs and ASDHs. The underlying cerebral injury can be a limiting factor in achieving desirable outcomes after adequate removal of ASDHs. Postoperative secondary injury should be avoided via controlling ICP.
- Manipulation or removal of small clots over large parasagittal draining veins or dural sinuses should be kept to minimum.
Contributor: Jonathan Weyhenmeyer, MD
References
Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58(3 Suppl):S16–24.
Leitgeb J, Mauritz W, Brazinova A, et al. Outcome after severe brain trauma due to acute subdural hematoma. J Neurosurg 2012;117:324-333.
Winston KR. Efficacy of dural tenting sutures. J Neurosurg 1999;91:180–184.
Please login to post a comment.



















Comments: