Extramedullary Spinal Cord Tumor
This is a preview. Check to see if you have access to the full video. Check access
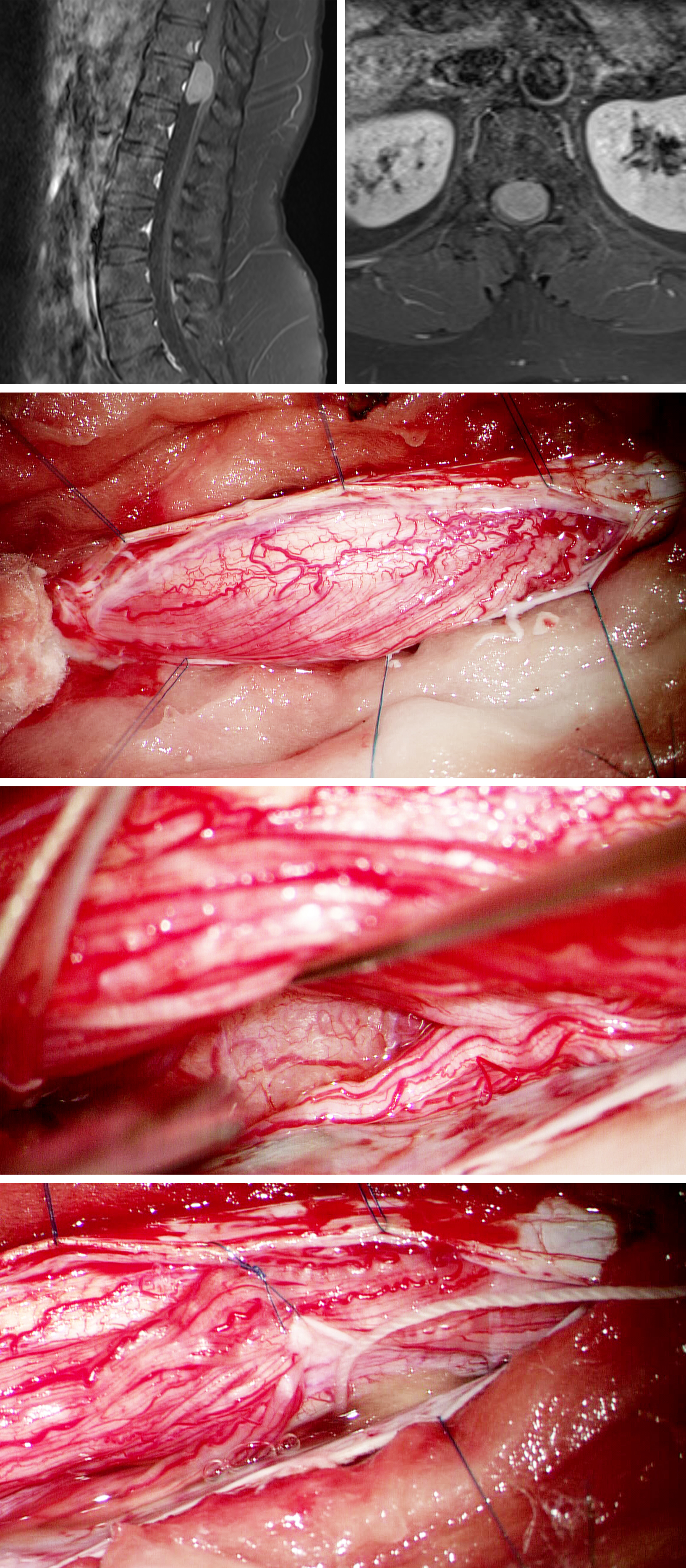
Resection of a Myxopapillary Ependymoma
Tumor Subtypes
Extramedullary primary tumors of the spinal cord, the spinal meninges, the cauda equina, and spinal nerves are relatively rare lesions that account for 3% of all central nervous system tumors and 4% of all spinal tumors.
Together, meningiomas, nerve sheath tumors, and ependymomas comprise 90% of all spinal cord tumors. Recent epidemiologic surveys indicate an estimated overall incidence of 0.74 to 1.5/100,000 person-years for these tumors during the MRI era. Approximately two-thirds of spinal cord tumors are reported as nonmalignant.
Extramedullary spinal cord tumors represent 42% to 67% of all tumors of the spinal canal. Most of these are exclusively intradural (~85%), whereas the remainder demonstrate some extradural extension. The most common histologic types of extramedullary spinal cord tumors are meningiomas (25%–46%), nerve sheath tumors (33.9%), and ependymomas of the filum terminale (5.6%–6.8%).
Arachnoid cysts, neurenteric cysts, dermoid cysts, meningeal melanocytomas, cavernomas, and hemangiopericytomas, as well as metastases, are also rarely reported.
For a detailed discussion about intramedullary tumors, please refer to the Intramedullary Spinal Cord Tumors chapter.
Meningiomas
Spinal meningiomas are usually benign, slow-growing, well-circumscribed lesions. They have a bimodal peak age distribution, separated at age 50. They typically affect elderly patients in the seventh decade of life (75%) and with a mean age of 63 ± 16.6 years. A second peak (25%) is also observed at the mean age of 34.5 ± 10.9 years (range, 9–49 years).
Younger patients have an increased occurrence of associated conditions, such as neurofibromatosis type 2, that portend a worse prognosis. Spinal meningiomas have a distinct female predominance with an approximate ratio of 3.5-5:1 (female/male).
Spinal meningiomas most commonly affect the thoracic spine (64-82%), followed by the cervical (14-28%), and lumbar (0-8%) spinal segments. Younger patients have an increased likelihood of tumors within the cervical spine (39%), mainly concentrated at the craniocervical junction.
Regarding the radial distribution of dural attachment, spinal meningiomas are commonly located anterolaterally (25%), laterally (30%), or posterolaterally (25%). Fewer than 10% of patients harbor anterior-based tumors. A circumferential or hemicircumferential disposition (en plaque) can be rarely found.
The most frequent presenting symptoms are sensory changes (80-87%), weakness (40-84%), and gait disturbances (68-83%). Back pain is a frequent complaint occurring in up to half of patients. At presentation, most patients are able to walk, especially those who are elderly.
Surgery often leads to good functional outcomes. Harvey Cushing stated that a “successful operation for a spinal meningioma represents one of the more gratifying of all operative procedures.” The Simpson grading score is not usually applied for spinal meningiomas because of the difficulty in radically resecting the affected dura.
Intraspinal Nerve Sheath Tumors
Intraspinal nerve sheath tumors are classified as schwannomas, neurofibromas, and malignant peripheral nerve sheath tumors (MPNST). Intraspinal schwannomas are mainly sporadic and may occur throughout the spinal canal. Occasionally, schwannomas may be associated with genetic disorders, such as neurofibromatosis type 2 and schwannomatosis.
Neurofibromas, however, are rarely sporadic and usually encountered in the spectrum of neurofibromatosis type 1. Intraspinal nerve sheath tumors have a relatively equal sex distribution (1.4:1; male:female ratio), and affect patients with a mean age of 47 years (range 5-85 years).
Schwannomas are the most common histologic type (92%) and are usually located within the cervical spine (27.5-41%), followed by the lumbosacral spine (30-55%), and the thoracic spine (17.5-29%). Exclusively intradural tumors are frequently encountered in the thoracic and lumbar spines, whereas intra-extradural (dumbbell-shaped) tumors are predominantly found in the cervical region. Neurofibromas comprise 7% of all intraspinal nerve sheath tumors and usually occur in the cervical spine.
Pain is the most dominating symptom (radicular pain in 69%, local pain in 68%), with a similar occurrence regardless of the extradural extension. Paraesthesia, weakness, gait disturbance, and bladder dysfunction are less frequently encountered.
Ependymomas (Filum Terminale and Extramedullary)
Extramedullary ependymomas are rare, slow-growing tumors that almost exclusively occur in the conus medullaris, cauda equina, and filum terminale regions. In these locations, extramedullary ependymomas are classified as myxopapillary ependymomas (MPEs; WHO grade I). MPEs arise from the ependymal lining of the spinal canal and typically affect young patients in their fourth decade of life.
Even more rare is the occurrence of extramedullary ependymomas in the thoracic or cervical region mimicking a spinal meningioma. In these situations, they arise from ectopic ependymal cells and are histologically WHO grades II or III.
Back pain dictates the clinical presentation (~90%). Weakness, sensory disturbances, and bladder dysfunction are usually observed. Their indolent course contributes to their considerable size at diagnosis, affecting 3.8 vertebral levels, on average. Spinal subarachnoid dissemination is a rare event. For large (greater than 4 levels) and recurrent tumors, the entire neuroaxis should be investigated with imaging.
Diagnosis
Most patients demonstrate a typical clinical course starting with pain of different characteristics, followed by radicular complaints and ultimately leading to progressive myelopathy. Acute deteriorations may be observed as a result of intratumoral hemorrhage or precipitated by a lumbar puncture.
Evaluation
Magnetic resonance (MR) imaging with contrast enhancement is the diagnostic study of choice for these tumors. Meningiomas, schwannomas, and ependymomas demonstrate similar signal characteristics on MR imaging, which makes them difficult to differentiate based on imaging alone. These tumors are commonly isointense on T1-weighted images and hyperintense on T2-weighted images. Contrast enhancement is similarly homogeneous.
Although meningiomas typically demonstrate a “dural tail,” this feature is not consistently seen. Enlarged neuroforamina is a common finding among nerve sheath tumors, but not exclusively, since it can also be observed in meningiomas with extradural extension. Cystic components are more likely to be associated with schwannomas and ependymomas, but less likely with meningiomas.
Figure 1: Typical imaging presentation of a cervical anterolateral meningioma: an intensely homogeneously enhancing dural-based tumor with a dural tail.
Figure 2: Typical imaging presentation of a cervical dumbbell-shaped schwannoma. Note the anterior displacement of the vertebral artery on the left side (asterisks) and the enlargement of the ipsilateral neuroforamina.
Figure 3: MR imaging of a myxopapillary ependymoma with solid and cystic components and intense heterogeneous contrast enhancement.
Computed tomography (CT) may be used, especially for investigating spinal anomalies related to neurofibromatosis and spinal dysraphism. Additionally, tumoral calcification can be evaluated. Spinal X-rays are not useful for diagnosing extramedullary tumors, but the occurrence of pedicular thinning or neuroforaminal expansion justifies further diagnostic investigation.
Indications for Surgery
Symptomatic tumors constitute the major surgical indication for their resection. Conversely, there is still controversy regarding the appropriate treatment plan for asymptomatic patients for whom surgery is considered prophylactic. In such instances, surgical decision-making is affected by the patient’s age, clinical status, associated comorbidities, and the patient’s preference.
Since meningiomas and schwannomas are slow growing, asymptomatic patients are usually scheduled for serial imaging, especially older patients. This is particularly true for patients with tumors associated with neurofibromatosis type 2 and schwannomatosis; these patients are usually affected by multiple lesions. Patients with symptomatic tumors are scheduled for surgery, whereas those with nonsyptomatic lesions are followed by annual imaging.
The exception to this treatment plan is a patient with a midline asymptomatic tumor of the cauda equina, potentially a myxopapillary epedymoma. Given that this tumor type can cause cerebrospinal fluid (CSF) dissemination, early surgery may be reasonable.
Radiosurgery may be used for small residual or recurrent tumors, for higher-grade meningiomas and for multiple lesions, as well as for palliation among patients unable to tolerate resection.
Preoperative Considerations
MR imaging should include T1-, T2-weighted, contrast-enhanced, and 3D-CiSS (constructive interference in steady state), or FIESTA (fast imaging employing steady-state acquisition) sequences in at least 2 planes.
CT angiography is rarely used for studying dumbbell-shaped tumors of the cervical spine and the relationship of the intraforaminal tumor to the vertebral artery. Digital subtraction angiography (DSA) is not necessary. The exception includes laterally seated tumors of the thoracic spine, when the position of the artery of Adamciewiecz should be clarified.
After diagnostic imaging, patients are classified according to their clinical symptoms and functional capabilities. The clinical parameters prognosticate their postoperative outcome. The main scoring systems for intradural extramedullary tumor are the Nurick scale and the Klekamp and Samii neurological score system.
| Grade | Description |
| 0 | Signs or symptoms of root involvement, but without evidence of spinal cord disease |
| 1 | Signs of spinal cord disease, but no difficulty walking |
| 2 | Slight difficulty walking that does not prevent full-time employment |
| 3 | Difficulty walking that prevents full-time employment or ability to do all housework, but that is not so severe as to require others’ help to walk |
| 4 | Able to walk only with a person’s help or with the aid of a frame |
| 5 | Chair-bound or bedridden |
| Grade |
Sensory Disturbance, Pain, Dysesthesias |
Motor Weakness | Gait Ataxia | Sphincter Function |
| 0 | Incapacitated function | Plegia | Plegia | Permanent catheter |
| 1 | Severe restriction of function | Contraction without movement | Standing with aid | Often catheter |
| 2 | Some restriction of function | Movement without gravity | Few steps with aid | Rarely incontinent |
| 3 |
Significant, function not restricted |
Movement against gravity | Mobile with aid |
Residual, no catheter |
| 4 |
Present, not significant |
Movement against resistance |
Unsteady, no aid |
Slight disturbance, no catheter |
| 5 | No symptom | Full power | Normal | Normal |
RESECTION OF EXTRAMEDULLARY SPINAL CORD TUMORS
Exposure
Use of an arterial line is advised during surgery for highly vascularized tumors; hypotension should be avoided to prevent cord hypoperfusion and ophthalmologic complications (anterior ischemic optic neuropathy and orbital compartment syndrome). Prophylactic antibiotic and dexamethasone are administered around the time of orotracheal intubation.
Intraoperative neurophysiologic monitoring of the major sensory and motor pathways is routinely used. These parameters include motor evoked potentials, somatosensory evoked potentials, free-running electromyography, and potentially direct nerve root stimulation. These metrics are also evaluated during patient positioning, especially for patients who harbor significant cervical spondylosis.
The patient is placed in the prone position over chest rolls with knees flexed. The bony prominences are padded. The semisitting position is an option for patients with cervical tumors, but it is not my preference.
The patient’s head is carefully positioned on a horseshoe headrest. For cervical tumors, the head is immobilized in a skull clamp and the neck flexed until the chin reaches a two-finger breadth distance to the chest. This maneuver increases the interspinous spaces of the cervical spine.
For most patients with extramedullary tumors, a posterior midline approach is all that is needed for safe exposure. I prefer a wide laminectomy, taking care not to disturb the capsule of the facet joint. Ligamentum flavum is removed all the way to the next bony segment cranially and caudally. Epidural hemostasis is obtained using gelfoam powder soaked in thrombin.
For lateral, anterolateral, and anterior tumors, a varying degree of facetectomy and pediculectomy is needed to avoid cord manipulation during tumor resection. Purely ventral cervical tumors very rarely require a 2 to 3-level corpectomy. Alternative anterior approaches are rarely indicated because tumor debulking provides adequate working space for its safe capsular mobilization.
The spinous processes are removed with a Leksell rongeur and a partial laminectomy is developed. A high-speed drill can be used to thin out the lamina; bone removal extends to the spinolaminar junction (medial to the facet joints). The profile of the Kerrison rongeur footplate should be at minimum to avoid secondary compression of the spinal cord during piecemeal laminectomy. A medial facetectomy and partial pediculectomy are necessary for anterior and anterolateral tumors.
An en bloc laminectomy is the preferred option for large space-occupying lesions that require multilevel exposure, especially those in the cervical spine. The insertion of rongeurs inside the spinal canal should be avoided. Trough laminotomies are done bilaterally with a high-speed drill (3-mm cutting burr). The troughs are made at the spinolaminar junction and deepened to the level of the dura. Next, the caudal interspinous ligament is divided and the most rostral and caudal spinous processes are elevated with Kocher clamps. Ligamentum flavum is cut with Kerrison rongeurs and the laminae are lifted en bloc. Finally, the interspinous ligaments are removed.
A footplate craniotome is a reasonable alternative for creation of the laminotomies, as long as the spinal cord is not under significant tension and the footplate will not place the tense spinal cord at risk via its vibrations. A small bone window is created at the lamina above and below the planned dural opening. The epidural space is identified and dissected. Then the footplate of the craniotome is placed inside the spinal canal at the spinolaminar junction bilaterally and used to complete the laminotomies. Thereafter, the operative steps are very similar to the ones mentioned in the above paragraph.
After standard bilateral laminectomies, further lateral dural exposure is secured through removal of the bone toward the spinolaminar junction. Immaculate epidural hemostasis is paramount to allow uninterrupted pristine microsurgery after dural opening.
Intraoperative ultrasound is a helpful adjunct for localizing the exact position of the tumor and the adequacy of the dural exposure before the dura is manipulated.
Figure 4: The dura is incised at the midline; an attempt is made initially to preserve the arachnoid layer. Retraction tack-up sutures are used to mobilize the dura laterally to increase the surgical exposure, as well as to reduce epidural venous bleeding.
Operative Approaches for Posterior and Lateral Tumors
Posterior and posterolateral tumors are readily identified after dural opening via expanded bilateral laminectomies extending to the spinolaminar junction. For tumors that are primarily lateral, a partial facetectomy and potentially even a partial pediculectomy may be needed to avoid retraction on the spinal cord during exposure and manipulation of the mass.
To further minimize the risk of traction injury during resective maneuvers, I aggressively debulk the tumor and its cystic components as early as possible in the dissection process. Thereafter, I use standard microsurgical methods to dissect the tumor capsule away from the spinal cord. Traction on the nondebulked tumor could be directly transmitted to the spinal cord, placing the already compromised cord at risk.
Posteriorly and laterally-seated meningiomas are debulked in a piecemeal fashion using an ultrasonic aspirator, pituitary rongeurs, and/or microscissors. If it is safe to do so, the tumor base should be devascularized early. Internal decompression is followed by disconnection of the tumor’s dural attachment. The tumor capsule is then mobilized away from the spinal cord microsurgically and into the resection cavity. Complete resection of the affected dura is not safe.
Operative Approaches for Anterolateral and Anterior Tumors
Anteriorly and anterolaterally-seated tumors are technically more demanding to remove, but they frequently provide adequate room for their safe resection through the posterolateral surgical route via preoperative displacement of the spinal cord.
Figure 5: In particular cases and for example in the cervical spine, the approach may be extended posterolaterally incrementally by removing the facet joint, pedicle, or even the lateral mass. Densely calcified tumors are initially approached through the posterolateral route. If resection is not deemed possible because of the need for spinal cord manipulation, the patient should be scheduled for an anterior approach at a later date. Extensive facet removal may require an arthrodesis procedure for spinal stabilization. The top sketch illustrates the use of aggressive bone removal on the right side for reaching the tumor without a need for extensive cord manipulation. The bottom illustration of the operative perspective depicts the use of pial/dentate ligament retraction sutures to gently mobilize the cord and further uncover the lesion.
An extended partial facetectomy is often adequate for resection of anterior lesions. The dura is incised in the midline and mobilized to one side with tack-up sutures, thereby expanding the posterolateral operative trajectory. Retraction sutures (6-0 prolene) in the dentate ligaments (after their release) can be used to gently and minimally rotate the spinal cord to further expand the operative trajectory.
Figure 6: In the thoracic spine, partial pediculectomy and retraction sutures on the dentate ligaments can provide an adequate operative corridor to resection of ventral and midline-based meningiomas.
Figure 7: Anterior meningiomas are generally hidden by the spinal cord (upper two rows of images) despite a partial pediculectomy. Mobilization of the roots makes the tumor somewhat accessible (image in the third row). If necessary, the spinal cord may be gently mobilized by placing sutures in the arachnoid or dentate ligaments, lightly displacing the cord (lower image). This maneuver should be conducted using intraoperative neurophysiologic guidance.
Figure 8: Internal decompression permits progressive tumor mobilization (upper image); gentle dynamic retraction on the cord is used. Anterior meningiomas can be mobilized completely only after dural detachment, which is widely coagulated after tumor removal (lower image).
Operative Approaches for Dumbbell Tumors
Dumbbell-shaped tumors demonstrate extradural extension beyond the intervertebral foramina. Bone removal should expose the extraforaminal component of the mass. A complete unilateral facetectomy is necessary on the side of the tumor. This extended posterior approach provides contiguous exposure of intradural, intraforaminal, and paraspinal tumor components up to 3 cm lateral to the dural root sleeve origin.
The dural opening should exposure the intradural, intraforaminal, and extraforaminal components of the tumor.
Microsurgical dissection of the arachnoid bands should reveal the proximal and distal sections of the affected nerve (afferent and efferent segments) relative to the tumor. Unsalvageable roots should be stimulated for any functional activity and sacrificed if it is safe to do so.
More commonly, however, the afferent and efferent tumor attachments are not visualized on initial tumor exposure. Progressive tumor debulking allows delivery of the tumor capsule into the resection bed until the afferent and efferent segments are identifiable.
The operator should remain judicious regarding the efforts used to dissect the nerves away from the tumor capsule. Although initially most of the nerves within the afferent bundle appear to be nondissectable from the capsule, patient microsurgical dissection often proves otherwise, and only a portion of the involved nerve fascicles need to be sacrificed for complete tumor removal. On the other hand, the surgeon should not insist on separating fascicles that are intimately involved with the capsule if their stimulation mapping reveals absence of function; this futile maneuver can unnecessarily lengthen the operation significantly.
Figure 9: Surgical exposure is directed more laterally over the affected neuroforamina by expanded bone resection using fine rongeurs or diamond drills. With exposure of the extradural part of the tumor, the dura is opened in a T-shaped fashion. A J-shaped incision along the nerve root is also an option.
Figure 10: The intradural portion is excised first. Next, the dissection is directed toward the extradural component. At this level, motor and sensory roots are usually so compressed that they are almost unidentifiable from the affected nerve sheath distally. Proximal nerve roots are stimulated and preserved if they demonstrate any motor response. Care is taken at the most lateral part of the extradural component because it is likely to be associated with the vertebral artery in cervical spine tumors. Large tumors with significant extradural extension may require a second-stage anterolateral approach for their radical removal.
Figure 11: Intraspinal nerve sheath tumors are generally positioned laterally within the spinal canal because they originate from the nerve roots. Schwannomas usually derive from sensory nerve roots. After initial preparation and internal debulking, the affected nerve sheath is identified and stimulated (black arrow). In there is no activation response, the root may be sacrificed along with the tumor capsule. If a motor response is found, the functioning nerve root should be preserved and radical tumor resection may not be possible. This conservative management strategy is followed especially with patients suffering from neurofibromatosis.
Spinal stability may be compromised in some patients, especially when significant removal of the lateral vertebral bone and facet joint occurs. In such cases, stabilization procedures may be necessary.
Operative Approaches for Cauda Equina Tumors
Extramedullary tumors located around the cauda equina can pose a significant surgical challenge because they can be attached to or engulf nerve roots.
Figure 12: Surgery for this L1 schwannoma follows the same concepts. The preoperative MR images are demonstrated in the first-row images, and the intraoperative ultrasonography image is included in the inset image. Laminectomy and midline dural incision exposed the schwannoma associated with a L1 rootlet. Stimulation revealed no evidence of function in the involved rootlet (second row). The associated nerve was therefore sacrificed and the tumor excised (bottom row).
Figure 13: Ependymomas (images in the first row) demand a different operative strategy. The first surgical step is the dissection of the nerve roots from the tumor capsule (upper image) to expose a reasonable portion of the capsule to allow internal decompression. En bloc resection, including the filum terminale, is advisable for small- and medium-sized tumors to protect against CSF dissemination. Larger tumors are debulked early in surgery. The surgical field should be covered with cottonoid patties to avoid subarachnoid seeding of tumoral cells. The filum terminale (asterisk) is identified on both tumor poles and then it is transected (lower image). Some roots of the cauda equina may be engulfed by the mass. Stimulation of the engulfed nerves will determine the feasibility of their transection for gross total resection.
Closure
The dura is closed with running sutures in a watertight manner. Duraplasty is necessary to repair the resultant dural defect if the dural edges cannot be readily approximated. Watertight dural closure is not feasible after resection of dumbbell-shaped lesions and fat globules may be used to plug the defects.
Extended laminectomies can lead to postoperative spinal deformity. Thus, larger tumors in pediatric patients may benefit from an osteoplastic laminotomy, which may potentially decrease the risk of such deformity.
Postoperative Considerations
The patient is typically observed on the regular floor for frequent neurologic evaluations, as well as pain and blood pressure control. A postoperative MR scan is obtained in select cases. Steroids are weaned slowly postoperatively. Patients should be treated with aggressive preventive measures for deep-vein thrombosis, including early mobilization.
Long-term follow-up for development of delayed spinal deformity, especially in cases that involved multilevel surgeries, is necessary.
Pearls and Pitfalls
- The most common histologic types of extramedullary spinal cord tumors are meningiomas, nerve sheath tumors, and ependymomas of the filum terminale.
- The typical clinical course starts with pain, followed by radicular complaints and ultimately progressive myelopathy.
- Most intradural extramedullary tumors may be resected using posterior and posterolateral midline approaches, regardless of the tumor position within the spinal canal.
Contributor: Marcus André Acioly, MD, PhD
References
Benzel EC: Neural element injury. In: Biomechanics of Spine Stabilization. Rolling Meadows: AANS Publications, 2001, p 94–95.
Cohen-Gadol AA, Spencer DD, Krauss WE. The development of techniques for resection of spinal cord tumors by Harvey W. Cushing. J Neurosurg Spine. 2005;2:92-97.
Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg. 2003;98(3 Suppl):258-263.
Feldman WB, Clark AJ, Safaee M, Ames CP, Parsa AT. Tumor control after surgery for spinal myxopapillary ependymomas: distinct outcomes in adults versus children: a systematic review. J Neurosurg Spine. 2013;19:471-476.
Halvorsen CM, Rønning P, Hald J, Johannesen TB, Kolstad F, Langmoen IA, Lied B, Skaar Holme S, Helseth E. The long-term outcome after resection of intraspinal nerve sheath tumors: report of 131 consecutive cases. Neurosurgery. 2015;77:585-593.
Kinsman MJ, Callahan JD, Hattab EM, Cohen-Gadol AA. Extramedullary spinal ependymoma: a diagnostic challenge and review of the literature. Clin Neurol Neurosurg. 2011;113:661-664.
Klekamp J. Spinal ependymomas. Part 2: Ependymomas of the filum terminale. Neurosurg Focus. 2015;39:E7.
Klekamp J, Samii M. Surgery of Spinal Tumors. 1st Ed. Berlin Heridelberg, Springer, 2007.
McCormick P. Intradural Extramedullary Tumors (Chapter 187), In Quinones-Hinojosa A (ed): Schmidek & Sweet Operative Neurosurgical Techniques, Vol 2, 6th Ed, Philadelphia: Elsevier Saunders, 2012, 2127-2133.
Sandalcioglu IE, Hunold A, Müller O, Bassiouni H, Stolke D, Asgari S. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035-1041.
Weber C, Gulati S, Jakola AS, Habiba S, Nygaard ØP, Johannesen TB, Solheim O. Incidence rates and surgery of primary intraspinal tumors in the era of modern neuroimaging: a national population-based study. Spine (Phila Pa 1976). 2014;39:E967-973.
Please login to post a comment.