Anatomy of the Ventricular System
This is a preview. Check to see if you have access to the full video. Check access
The Rhoton Collection: Navigating the Ventricles
The discussion regarding the operative anatomy of the ventricular system is divided into three parts:
- Lateral Ventricle Anatomy
- Third Ventricle Anatomy
- Fourth Ventricle Anatomy
LATERAL VENTRICLE ANATOMY
Anatomically, the lateral ventricle can be thought of as a C-shaped capsule encompassing the thalamus and diencephalon. It is partitioned into five segmental divisions, which hold important distinctions during consideration of surgical approaches.
These five divisions are the anterior (frontal) horn, body, atrium (trigone), temporal horn, and occipital horn. For a more detailed description of the lateral ventricular segments, see the discussion in the Principles of Intraventricular Surgery chapter.
An interface exists between the lateral and the third ventricles via the foramen of Monro. This anatomic bottleneck is bordered by the septum pellucidum, corpus callosum, caudate nucleus, thalamus, and the fornix.
A thorough understanding of the ventricular anatomy is imperative for successful surgery within this region.
Click here to view the interactive module and related content for this image.
Figure 1: The corpus callosum is the largest anatomic interface with the lateral ventricle. This structure is divided into four segments. From anterior to posterior, these are the rostrum, genu, body, and splenium (image courtesy of AL Rhoton, Jr).
The fornix is a significant anatomic structure to consider during intraventricular surgery. It primarily contains projection fibers connecting the hippocampus to the hypothalamus. The pathway of the fornix begins in the hippocampal alveus, proceeds to the fimbria, and advances adjacent to the temporal horn of the lateral ventricle. Its function in memory should not be underestimated. Any operative intervention should consider the health and importance of this vital structure.
The forniceal bodies form the anterior and superior margins of the foramen of Monro. Any interforaminal procedure should consider the proximity of the fornices.
Click here to view the interactive module and related content for this image.
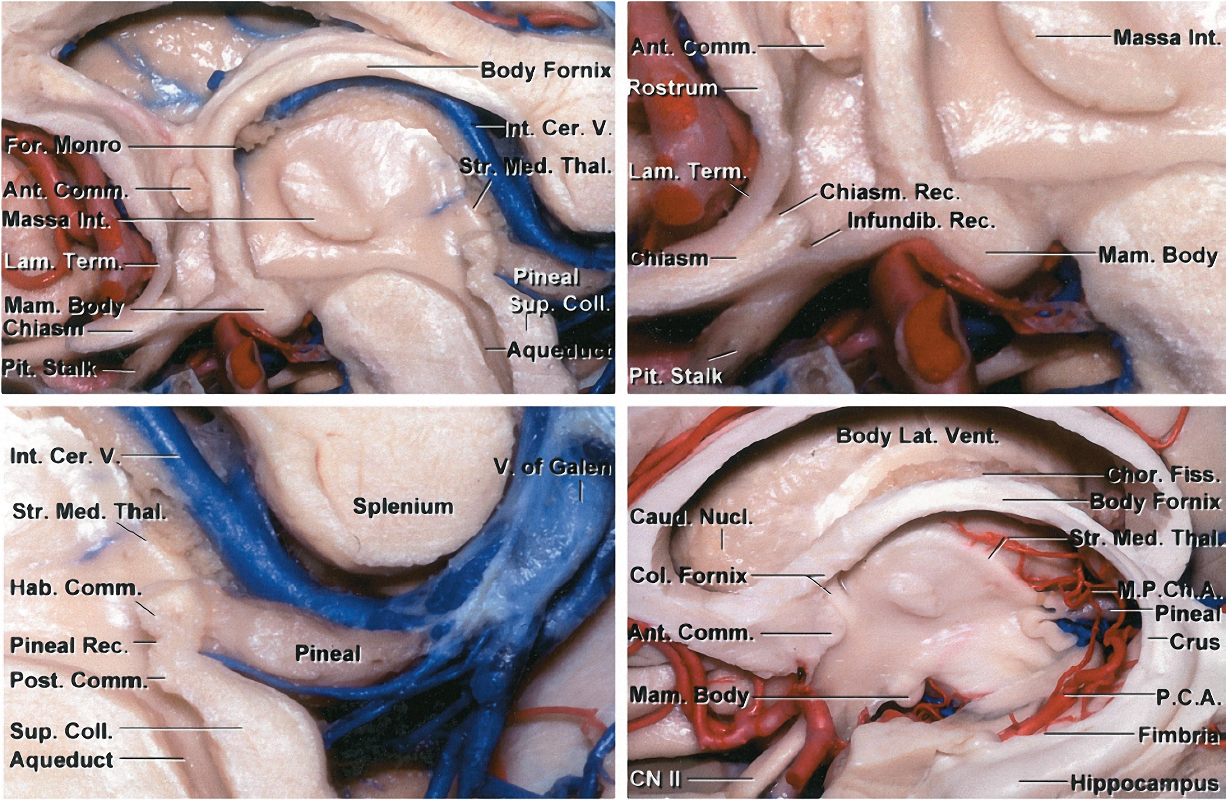
Figure 2: As the bilateral fornices curve along the periphery of the thalamus, they both merge along the rostral surface of the thalamus to form the body of the fornix. The fused forniceal bodies proceed along the periphery of the thalamus until they splits adjacent to the foramen of Monro as columns of fornix. These now separate structures project to the hypothalamus and mammillary bodies (left image). In the right image, the right fornix was transected to uncover the underlying structures along the choroidal fissure. During the transchoroidal route, I prefer to conduct dissection on the thalamic side of the choroid rather than the forniceal side to protect the fornix. However, this is not always the case because the anatomy of the fissure can also guide the more suitable side of dissection (M.P.Ch.A: Medial posterior choroidal arteries) (images courtesy of AL Rhoton, Jr).
The striatum is also a key anatomic structure. The primary structure of interest is the caudate nucleus.
Click here to view the interactive module and related content for this image.
Figure 3: The caudate is anatomically segmented into three divisions: head, body, and tail. It is vulnerable during intraventricular surgery because it passes deep to the lateral border of the anterior horn and body of the lateral ventricle. It is also deep to the roof of the temporal horn (images courtesy of AL Rhoton, Jr).
The genu of the internal capsule approaches the ventricular surface and directly touches the wall of the lateral ventricle immediately lateral to the foramen of Monro. This occurs in the interval between the caudate nucleus and the thalamus.
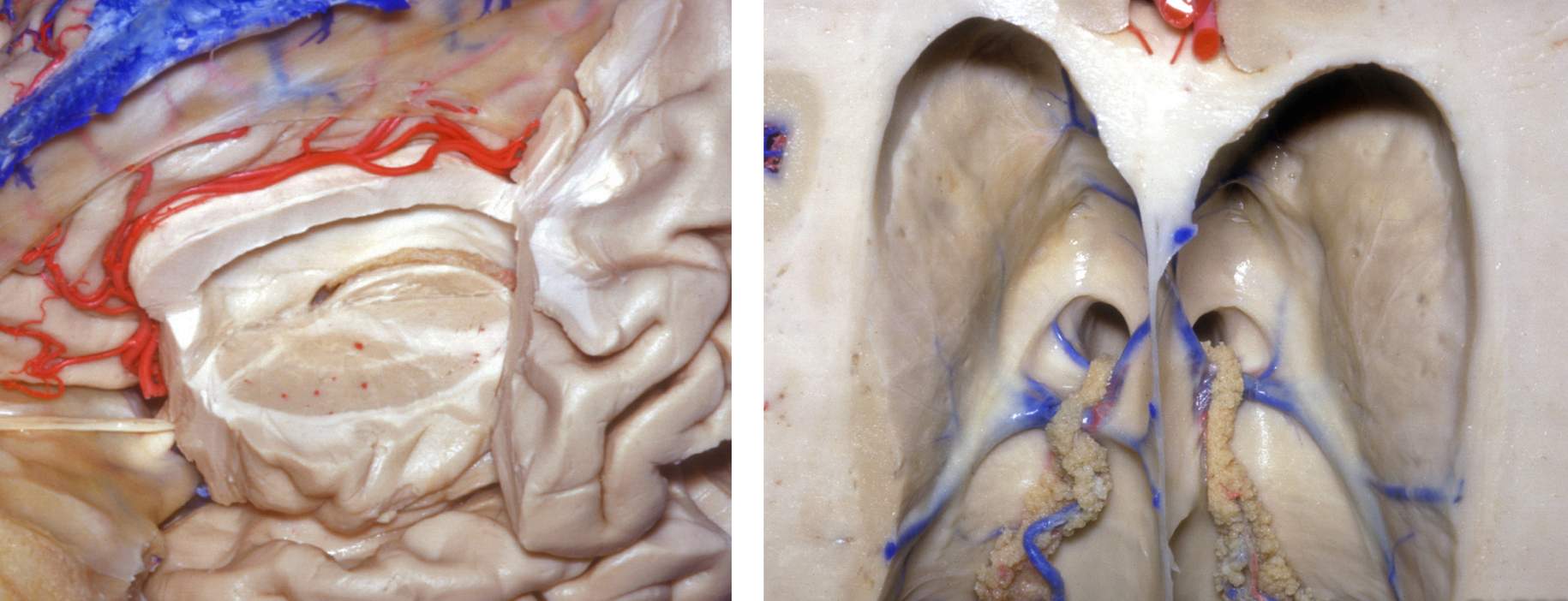
The choroidal structures are pertinent during planning of the resection and early devascularization of the feeding vessels to the tumor. The choroid plexus adheres to the choroidal fissure along the medial wall of the lateral ventricle. The choroidal fissure represents the separation between the fornix and thalamus and originates adjacent to the foramen of Monro, coursing along the lateral ventricular body, atrium, and temporal horn. The relationship of the fissure to the thalamostriate vein provides a reliable landmark for identifying the side of the lateral frontal horn.
Figure 4: The operative anatomy near the choroidal fissure is depicted (upper images). Transection of the septal vein as it joins the thalamostriate vein (*) is safe and allows an expanded transforaminal route toward the third ventricle with minimal dissection of the anterior choroidal fissure. This maneuver avoids significant manipulation of the fornix and thalamus required for a purely transchoroidal trajectory (right upper image). The indispensable thalamostriate vein must be preserved (images courtesy of AL Rhoton, Jr).
Figure 5: Expanded view of the transchoroidal route, the third ventricle and internal cerebral veins is demonstrated (left image). The interforniceal pathway provides generous exposure of the third chamber (right image), but the risk of retraction injury to the bilateral forniceal bodies is significant (images courtesy of AL Rhoton, Jr).
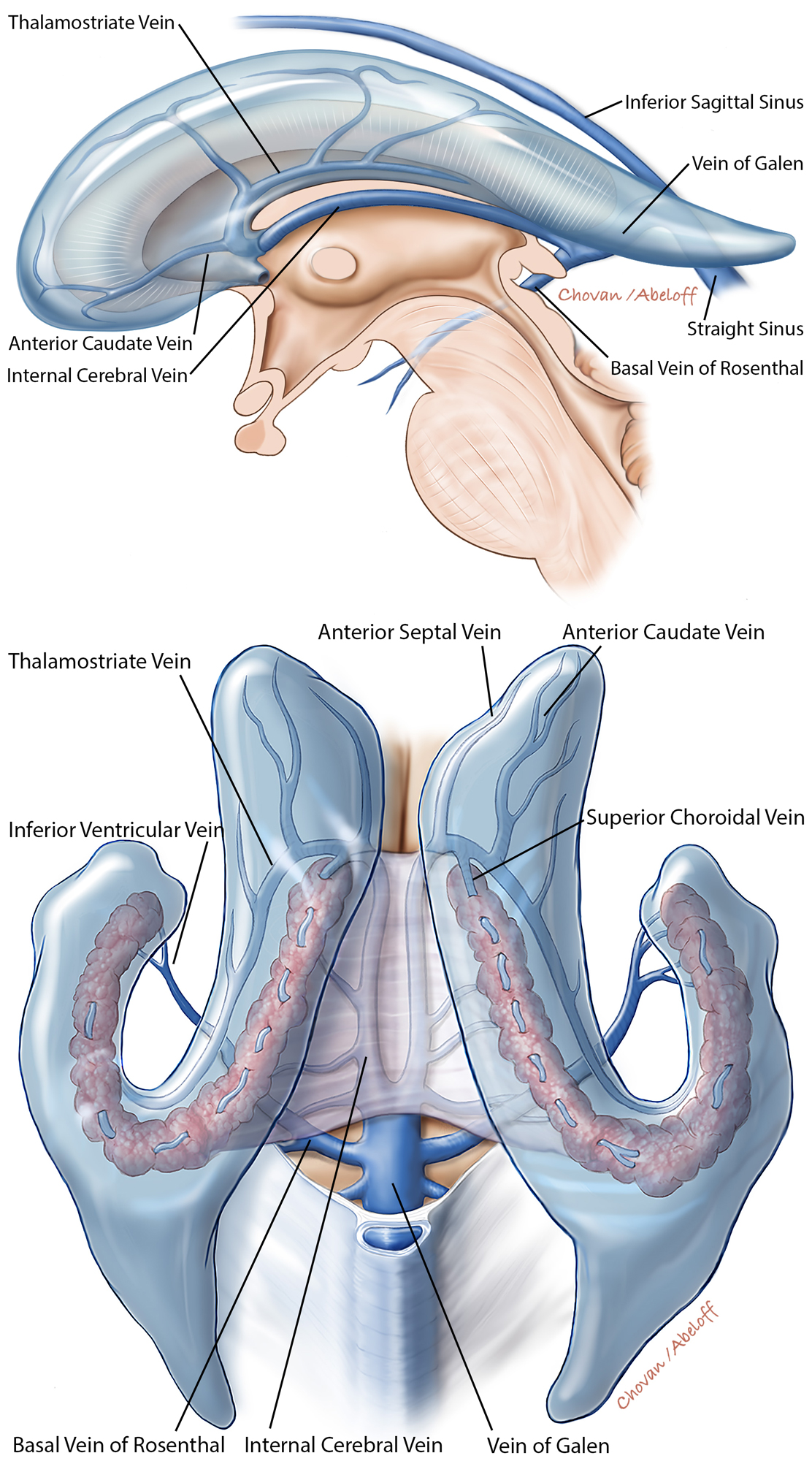
The key vascular anatomy to consider during lateral ventricular surgery includes: the anterior and posterior choroidal arteries (perfusing the choroid plexus), caudate and anterior septal veins, superior choroidal vein, medial and lateral atrial veins, thalamostriate vein, inferior ventricular vein, and inferior choroidal vein. The major draining veins include the internal cerebral veins and the basal veins of Rosenthal.
Figure 6: The ventricular venous anatomy is further illustrated in the above illustrations (sagittal, top, and superior, bottom, views). The veins especially associated with operative procedures are marked.
THIRD VENTRICLE ANATOMY
Familiarity with the anatomy of the deep diencephalic nuclei and midline ventricular system is vital for successful access and manipulation of third ventricular tumors. The third ventricle serves as a passageway from the lateral ventricles to the fourth ventricle. The interfaces between the lateral, third, and fourth ventricles are the foramen of Monro and aqueduct of Sylvius, respectively.
The regional anatomy of the third ventricle may be divided into anterior, posterior, lateral, cranial (roof), and caudal (floor) borders. These borders are described in that order below.
Click here to view the interactive module and related content for this image.
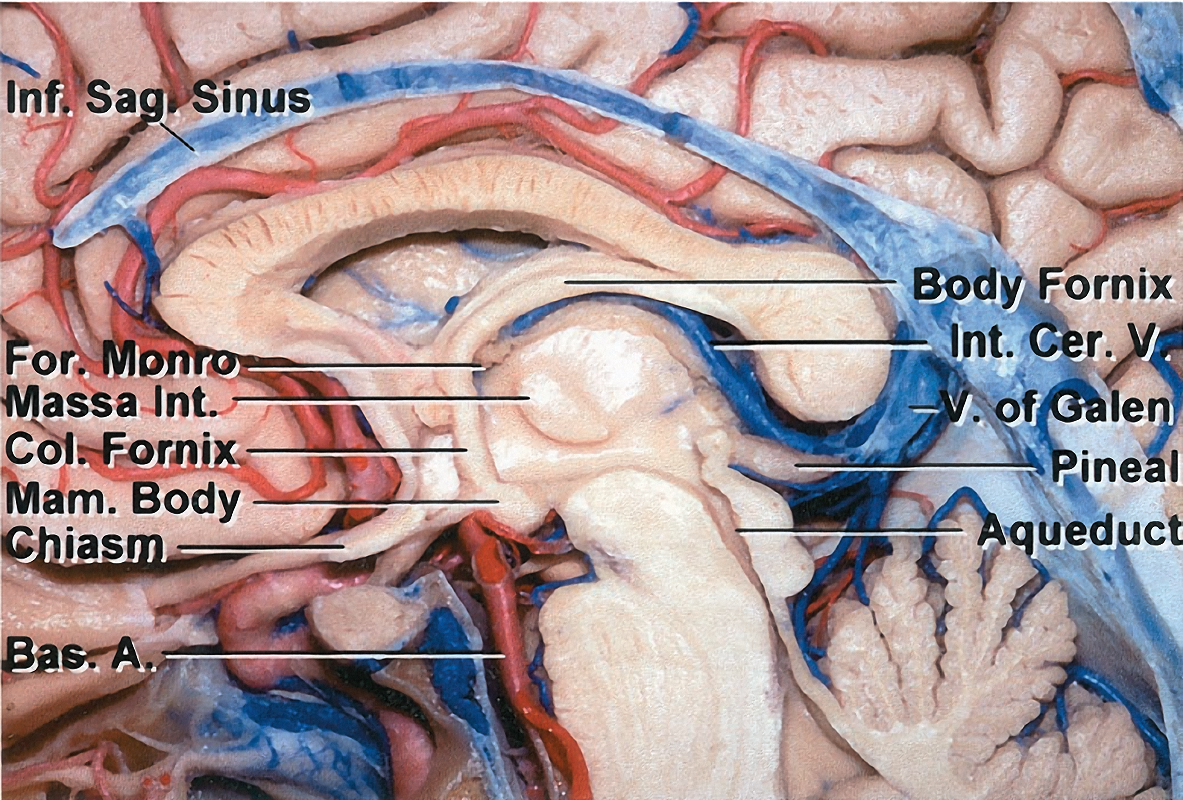
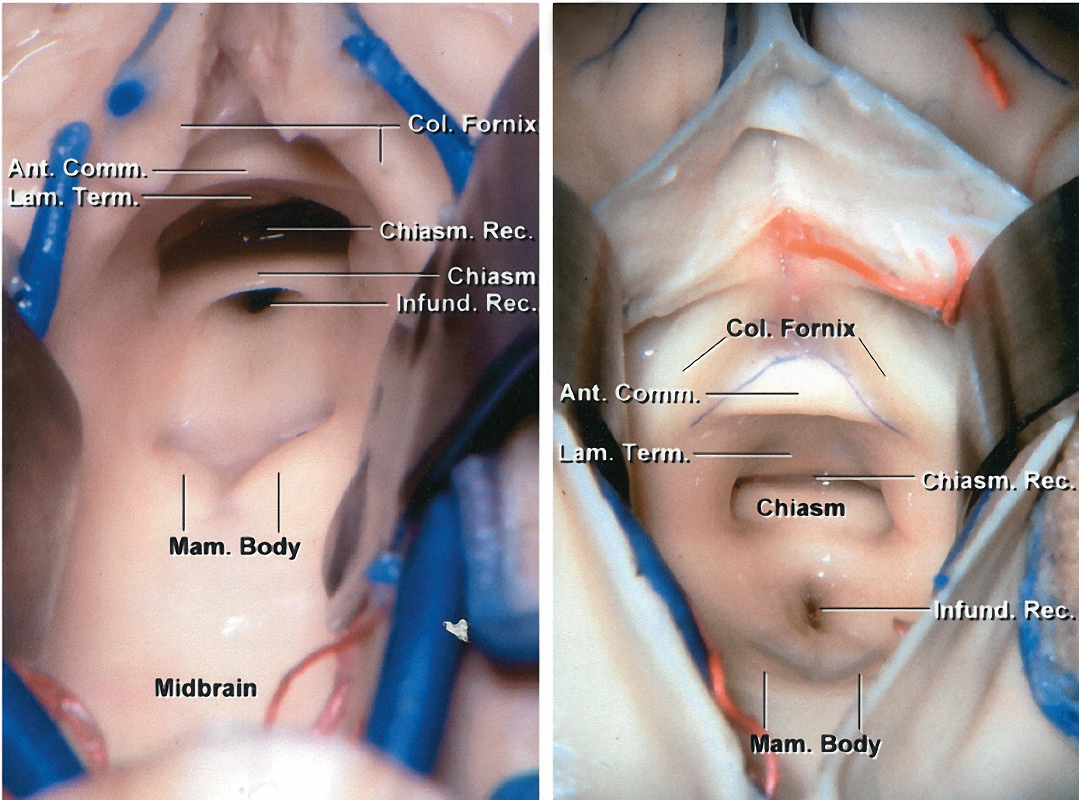
Figure 7: The anterior border of the third ventricle is composed of the region between the optic chiasm and the foramen of Monro. The structures in this region include the optic chiasm, lamina terminalis, anterior commissure, and columns of the fornix. The posterior border of the third ventricle is composed of the region between the aqueduct of Sylvius and the suprapineal recess. The structures composing this region include the posterior commissure, pineal body, and habenular commissure (images courtesy of AL Rhoton, Jr).
The lateral border of the third ventricle includes the thalamus and hypothalamus. The massa intermedia, the interconnection between the adjacent lateral walls of the third ventricle, is present in approximately 75% of the population. The roof is bordered superiorly by the foramen of Monro and extends to the suprapineal recess.
The roof is composed of five distinct structural planes. In order of deep to superficial, they include: a choroid plexus layer, a tela choroidea layer, a vascular layer (velum interpositum) composed of the medial posterior choroidal arteries and internal cerebral veins), an additional tela choroidea layer, and a forniceal layer composed of the fornix (see Figure 2 above).
Click here to view the interactive module and related content for this image.
Figure 8: The floor of the third ventricle is composed of a region from the aqueduct of Sylvius to the optic chiasm. The structures that make up this border include the posterior perforated substance, mammillary bodies, tuber cinereum, and infundibulum (also see Figure 6 above)(images courtesy of AL Rhoton, Jr).
The venous angle, composed of the anastomosis of the thalamostriate and the anterior septal veins, is located along the posterior edge of the foramen of Monro. The venous angle extends 3 to 7 mm beyond the posterior margin of the foramen of Monro in 30% of the population; this anatomic configuration can facilitate access to the third ventricle through the foramen.
The internal cerebral veins are situated at the midline during the posterior transcallosal approach to the pineal recess. Upon reaching the pineal recess, the path of the internal cerebral veins extends along the superolateral aspect of the pineal body. The internal cerebral veins unite inferior to the splenium to form the vein of Galen. Large pineal region tumors often create operative space between (intervenous) or around (paravenous) these veins for the posterior transcallosal surgical corridor to be feasible.
Surgical manipulation of the intact floor and walls of the third chamber is associated with significant morbidity. Subtotal resection of the infiltrating tumors is advised.
FOURTH VENTRICLE ANATOMY
The major structures that compose the borders of the fourth ventricle along its craniocaudal extent are:
- Anterior (floor)—midbrain, pons, medulla
- Lateral—superior, middle, inferior cerebellar peduncles
- Superior (roof)—superior medullary velum, cerebellar lingula, fastigium
- Inferior (roof)—choroid plexus, tela choroidea, inferior medullary velum, cerebellar uvula and nodulus
Tumors of the fourth ventricle commonly originate from the fourth ventricular floor, choroid plexus, or tela choroidea. Other lesions may arise outside the ventricle, but extend into the ventricle, including medullary, tectal, and cerebellar hemispheric masses. These lesions secondarily expand into the ventricle and are thus accessible via the telovelar approach.
The telovelar approach is the most flexible and practical approach to the fourth ventricle. For further details, refer to the chapter on Telovelar Approach.
Click here to view the interactive module and related content for this image.
Figure 9: The basic principles of the telovelar approach are described. Note the path of dissection medial to the tonsil and lateral to the vermis. A wide exposure of the ventricle is possible through an inferior to superior trajectory after transection of the inferior medullary velum (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 10: The topography of the eloquent fourth ventricular floor is mapped. The locations of the facial colliculus, as well as the hypoglossal and vagal triangles, are evident. Stimulation mapping can effectively guide the surgeon to avoid these critical structures during surgery of the floor (images courtesy of AL Rhoton, Jr).
The vascular structure most pertinent to the telovelar approach is the posterior inferior cerebellar artery (PICA). The PICA has five segments: anterior medullary (P1), lateral medullary (P2), tonsillomedullary (P3), telovelotonsillar (P4), and the cortical (P5) segments. The naming of these segments is not surgically relevant as long the operator appreciates the importance of the first three segments in giving rise to brainstem perforators.
Knowledge of the anatomic distribution of these segments is critical to the surgeon’s successful navigation of the fourth ventricle. The first three branches listed are also of particular importance as they are the predominant feeding vessels for fourth ventricular tumors. Tumors may also involve the vasculature of the choroid plexus and/or tela choroidea.
Click here to view the interactive module and related content for this image.
Figure 11: The morphology of the PICA from different views is depicted in these photos. The first three major PICA segments, namely the anterior medullary (P1), lateral medullary (P2) and tonsillomedullary (P3) segments, also provide arterial supply to the brainstem. Any segment of the PICA in close proximity of the brainstem can provide vascular support to the brainstem and should be preserved (images courtesy of AL Rhoton, Jr).
Up to 20% of PICAs originate from the vertebral artery extradurally. This anatomical variant should be considered during the extradural dissection of the vertebral artery at the craniocervical junction. The caudal loop of PICA encompasses the segment of the PICA between the lower cranial nerves and the pole of the tonsil. The cranial loop of the PICA courses between the rostral pole of the tonsil and the inferior medullary velum.
Pearls and Pitfalls
- The neurovascular anatomy of the corpus callosum is pertinent to surgery of the lateral ventricles.
- Similarly, the vascular anatomy of the foramen of Monro and choroidal fissure, as well as the fornix, is important for reaching the third ventricle.
- The telovelar approach provides flexible exposure of the fourth ventricle. The floor of this chamber should not be manipulated; appropriately selected operative intrinsic lesions of the region demand intraoperative functional mapping of the floor.
Contributor: Benjamin K. Hendricks, MD
Please login to post a comment.