Endoscopic Expanded Transnasal Approach
Figure 1: Anatomy of the anterior skull base seen through the transnasal endoscopic route (image courtesy of AL Rhoton, Jr).
Endoscopic transnasal surgery has revolutionized our operative philosophy for many challenging and difficult-to-reach skull base tumors. An understanding of the technical nuances of approach or “how to get there” is important for the skull base neurosurgeon to work as part of the team with the rhinologist to ensure adequate exposure for the tumor is secured. Overall, the transnasal approach remains in the territory of our rhinology colleagues.
I will review the nuances of the technique for the expanded transnasal exposure to the anterior skull base in this section. Suprasellar meningiomas, craniopharyngiomas, and other third ventricular lesions are suitable candidates for this approach.
I prefer to remove moderate size olfactory groove meningiomas (with the goal of saving preoperative functional olfaction) through a supraorbital craniotomy and an eyebrow incision. Although some colleagues have used the transnasal corridor for most olfactory groove meningiomas, the opportunity for saving olfaction is not feasible via this route, especially for moderate size meningiomas. Large and giant meningiomas are removed through an extended pterional craniotomy. I do not recommend the use of a bifrontal craniotomy.
ENDOSCOPIC EXPANDED TRANSNASAL APPROACH
Following induction of general anesthesia, preoperative antibiotics and a stress dose of methylprednisolone/dexamethasone are administered.
For routine pituitary tumor operations, I do not use a lumbar drain. However, I routinely place a lumbar drain for expanded skull base osteotomies for intradural non-adenomatous tumors. I clamp the drain for the duration of the operation and may use it for intrathecal administration of fluorescein. The patient’s head is placed either on a donut gel rest or in rigid pin fixation with the patient’s neck slightly extended and the head raised just above the level of the heart. Frameless stereotactic neuronavigation is registered using preoperative computed tomography (CT) and magnetic resonance imaging (MRI). A fascia lata donor site and abdominal fat donor site are prepared in anticipation of cranial base reconstruction. Routine nasal mucosal preparation is performed.
Following disconnection of the nasoseptal flap using monopolar electrocautery and its mobilization using blunt dissection, the mucosal side of the septal vascularized flap is pushed into the nasopharynx until tumor resection is complete, with the operator taking care to prevent twisting of its pedicle and resultant ischemia. The operation proceeds with a binostril technique in which one surgeon works bimanually while another drives the endoscope, using dynamic visualization to facilitate 3-dimensional perception of the surgical field. The use of an endoscope holder is an alternative technique, but not preferred since it prevents dynamic motion of the scope for advanced visualization.
The initial phase of the approach consists of lateral mobilization of the middle and inferior turbinates bilaterally, identifying the sphenoid ostia, performing a posterior septectomy, creating a wide sphenoidotomy, removing the sphenoid mucosa, performing partial posterior ethmoidectomies, and drilling the bony septations in the sphenoid sinus. The middle turbinate may be removed on one side to accommodate the endoscope to prevent its interference with manipulation of the instruments, but often the turbinates can be preserved. The above steps are tailored based on the underlying pathology.
A wide sphenoidotomy is important for the remainder of the operation because it allows greater degrees of operative freedom for passage and manipulation of instruments in the deep operative fields above the chiasm, and it minimizes the potential for instrument/endoscope collision or “sword fighting.” If both opticocarotid recesses cannot be readily seen with a 0-degree scope, the lateral exposure is inadequate. Variations in septal (septum deviation) or sphenoid sinus (multiseptated) anatomy can affect the extent of the septectomy and sphenoid sinus resection.
The lateral aspects of the surgical corridor should be limited by the nasal turbinates rather than by remnants of the anterior sphenoid wall. Posterior ethmoidectomy fully exposes the planum sphenoidale, and although the lesion may not extend to this level, this maneuver expands the operative corridor, widens the working angles, and prevents overhanging bone from impeding visualization and manipulation of instruments.
Complete removal of the sphenoid mucosa prevents formation of a postoperative mucocele, reveals important bony landmarks necessary for subsequent steps in the procedure, and provides the necessary bony substrate for adhesion of the pedicled nasoseptal flap.
All the above steps, including the elevation of the nasoseptal flap, are not necessary for common pituitary tumors and posterior ethmoidectomy has to be tailored for the individual tumor.
Click here to view the interactive module and related content for this image.
Figure 2: The medial opticocarotid recess is an important landmark because it represents the ventral aspect of a pneumatized middle clinoid process. It also marks the medial aspects of the parasellar carotid canal and the cavernous sinus, the lateral edge of the sella, and the inferomedial aspect of the optic nerve. The medial opticocarotid recess is the most lateral extent of the tuberculum sellae, and removal of bone over this landmark widens intradural exposure and allows the surgeon to work from normal anatomy toward pathologic anatomy by early identification of the optic nerves and the paraclinoid internal carotid artery (ICA), followed by visualization of the opticocarotid cistern and the supraclinoid ICA (Car., carotico, carotid; LOCR, lateral opticocarotid recess; Med., medial; MOCR, medial opticocarotid recess; Opt., optico; Prom., prominence; Pt., point; Rec., recess; Tuberc., tuberculum)(images courtesy of AL Rhoton, Jr).
There is osteological and morphological variation along the floor of the parasellar skull base. Unfortunately, the medial opticocarotid recess is not always visible, so the lateral opticocarotid recess and bony prominences over the carotid artery can be used to estimate the location of the medial opticocarotid recess. The sella is usually easily identified between the bilateral carotid artery prominences. The clival recess is visible inferior to the sella. If the sphenoid sinus is not well pneumatized, as in young children, these landmarks may be quite difficult to recognize, necessitating sole reliance on navigation and careful thinning of the bone until the landmarks can be reliably established.
A high-speed diamond-bit drill (or an ultrasonic curette) is used to remove bone over the sella turcica, tuberculum sellae, and posterior portion of the planum sphenoidale. Bone removal extends laterally to the medial opticocarotid recesses. The bone is initially shelled out or thinned using the drill and then removed using a Kerrison rongeur. I use copious amount of irrigation while drilling over the medial opticocarotid recesses to prevent thermal injury to the optic nerve. These nerves can be displaced and easily injured if not looked for and protected at all times.
Click here to view the interactive module and related content for this image.
Figure 3: Endonasal osteotomy over the sellar wall. The dura mater over the carotid arteries has been partially resected. The medial opticocarotid point (marked with yellow arrows) marks the middle clinoid. This point is an important landmark if it is identifiable before the start of the bone work (images courtesy of AL Rhoton, Jr).
Venous bleeding is often encountered upon removal of bone in this area, but even vigorous venous bleeding is usually easily controlled with gelfoam packing and gentle pressure. I use an ample amount of Floseal hemostatic matrix (Baxter, Deerfield, IL) to seal the bleeding from the cavernous sinus and other venous lakes in the exposed dura. The extent of bony exposure in the sagittal plane is determined by the size and location of the tumor along the same plane, and can be guided intraoperatively by using neuronavigation.
Tumors confined to the sella require removal of the anterior sellar wall, whereas preinfundibular tumors within the suprasellar cistern require more bony removal over the tuberculum sellae and the planum sphenoidale and less removal of the inferior/anterior sellar wall. However, it is recommended that the operator removes the bone above and below the superior intercavernous sinus to control and transect this vascular structure in order to open the diaphragma sella, which lies just behind this sinus.
Transinfundibular tumors often extend superiorly into the anterior third ventricle, and their exposure requires excision of additional bone over the anterior sella to accommodate the steeper working angles required to reach the superior ventricular extent of these lesions. Retroinfundibular tumors necessitate removal of the sellar floor along the inferior intercavernous sinus and occasionally along the posterior clinoid processes and dorsum sella.
More aggressive skull base osteotomies allow exposure and resection of tumors extending from the infundibulum into the prepontine and interpeduncular cisterns, as well as lateral transposition of the pituitary gland as needed. Alternatively, an “above and below” approach may be performed to reach both above and behind the sella. Transclival approaches require variable clivectomy based on the breadth of the tumor.
The distinctions among the tumor types mentioned above and in the following chapters are not absolute because most tumors occupy more than one of these anatomic compartments. The anterior extent of bony opening along the posterior planum sphenoidale does not need to expand significantly since most dissection, especially in the case of a craniopharyngioma, is carried out beneath the chiasm. However, if the tumor extends markedly above the chiasm, requiring a translamina terminalis approach, additional bone resection along the planum may be needed to allow a more direct working angle to the region.
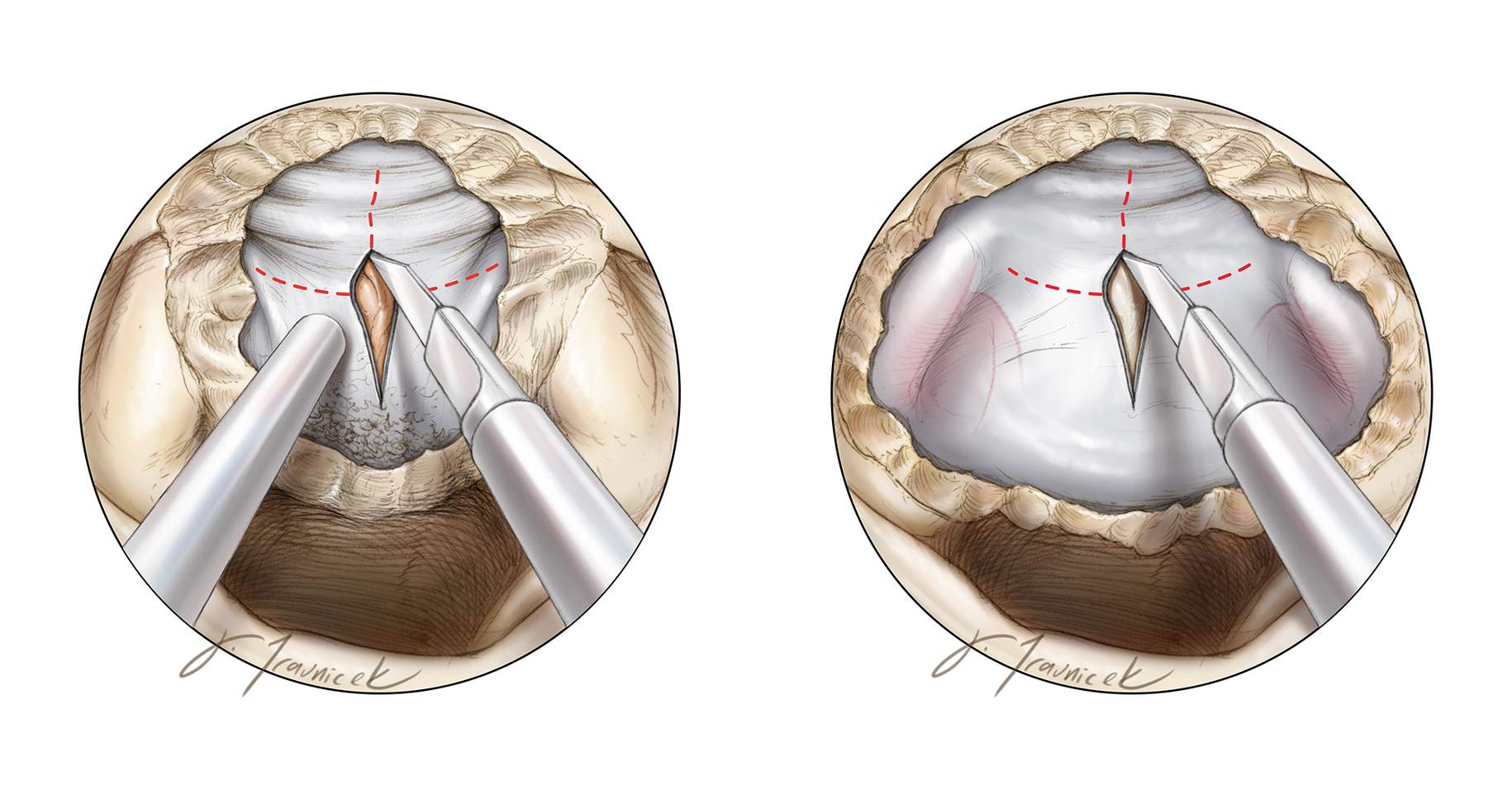
Figure 4: Sphenoidotomy and mucosal removal should include a posterior ethmoidectomy to visualize the posterior planum sphenoidale, the suprasellar notch, and the full anterior wall of the sella, which requires removal of the rostrum flush with the sphenoid sinus floor. Mucosal removal should extend laterally to the level of the lateral opticocarotid recesses. This exposure is necessary for intradural anterior skull base tumors.
A high-speed drill with a round bit is used to remove the bone of the suprasellar notch, inferiorly down the anterior wall of the sella, based on the extent of the tumor, and anteriorly along the posterior planum until the anterior limit of the tumor is reached. Neuronavigation provides additional guidance for the extent of the osteotomy. For most tuberculum sella meningiomas, I remove only the anterior half of the rostrum; this minimal bone removal protects the pituitary gland and facilitates a watertight skull base reconstruction at the end of tumor resection.
Lateral bone removal extends to the level of the medial opticocarotid recesses. If the optic canal is involved radiographically by the meningioma, the medial optic canal is unroofed over the extent of tumor. Bone removal should encompass the edges of the tumor’s dural attachments (left image). I remove the bone over the carotid arteries to avoid blind dissection around the lateral edges of the tumor capsule, especially in the case of a craniopharyngioma extending laterally beyond the boundaries of the sella (right image).
Most tumors of the paraclival area (chordomas and chondrosarcomas) affect the clivus and bony removal is centered over the affected area. Navigation based on a preoperative CT angiogram guides the osteotomy while the carotid artery is carefully localized.
Following adequate bone removal as confirmed by navigation, the superior intercavernous sinus is coagulated, and the dura is opened in a cruciate fashion. The dural edges are cauterized and shrunk to increase visualization. It is also possible to excise the dural edges using Kerrison rongeurs to expand the operative corridor. Before dural opening, microdoppler ultrasonography is routinely used to avoid injury to the ICA, especially since its proximal supraclinoid segment courses medially.
Figure 5: The dura is opened in a cruciate fashion, and resected where involved with tumor (left image). Since most craniopharyngiomas are cystic, the anteroposterior extent of osteotomy and the dural opening can be smaller than the size of the lesion because early cyst drainage leads to a dramatic decrease in the size of the mass and only the nodule needs to be microsurgically dissected (right image).
During later steps of the exposure and subsequent resection, use of a 30-degree angled endoscope may be preferable so that the tip of the endoscope may be moved out of the working zone of the surgical instruments while the operator maintains adequate visualization.
The details of intradural microsurgery for parasellar/tuberculum sella meningioma and suprasellar/third ventricular craniopharyngioma are discussed in the following chapters.
Closure
The exposure and osteotomy should be conducted with the plans of closure in mind. Cerebrospinal leakage remains one of the unconquered challenges in transnasal skull base surgery.
Figure 6: Once tumor resection is complete and the involved dura has been resected and/or cauterized, a gasket closure technique is used by first covering the bony defect with an oversized dural substitute or fascial graft, followed by a countersunk rigid implant: Porex (Stryker, Kalamazoo, MI). Depending on the lateral extent of the bony opening, notches are cut into the implant to avoid contact with the optic nerves.
I do not pack the suprasellar space or tumor cavity with foreign material in order to avoid unintended optic nerve compression and facilitate interpretation of postoperative images.
If the bony defect is large and not amenable to the gasket closure technique, a different closure plan is feasible. A layered closure using harvested or allograft dural reconstruction materials may be used, including intradural placement of dural substitute and extradural placement of the nasoseptal flap. Based on the preference of the rhinologist, various materials may be used to pack the nose and buttress this construct.
Valsalva maneuvers inspect for any obvious CSF leakage before the next step in reconstruction is attempted. The nasoseptal flap is then placed over the preferred method of initial closure so that the flap is in direct apposition to the surrounding bony skull base and subsequently held in place with DuraSeal (Covidien, Dublin, Ireland) or fibrin glue.
Bilateral nasoseptal flaps can also be used for larger skull base defects. Floseal (Baxter, Deerfield, IL) is administered to stop bleeding from mucosa. We insert a gelfoam sponge followed by nasal tampons to buttress the closure and limit postoperative nasal discharge.
Postoperative Considerations
The patient is observed in the intensive care unit overnight for frequent neurologic evaluations and pain/blood pressure control. I open the lumbar drain on the first postoperative day and remove 5-10cc/hour. The lumbar drain is discontinued ~2-4 days after surgery to facilitate early mobilization. A postoperative MRI is obtained.
Steroids may be slowly weaned as tolerated by the patient. Prophylactic anticonvulsants may be administered perioperatively, but tapered off 1 week after surgery if the patient has not suffered a seizure. Routine postoperative antibiotics are continued while the drain is in place. Monitoring of urine output and serum sodium allows management of temporary postoperative diabetes insipidus. Standard endonasal precautions are recommended to the patient, including avoidance of nose-blowing, straw use, and unnecessary bearing down.
Pearls and Pitfalls
- A thorough understanding of the parasellar skull base anatomy is important for safe completion of the bone work.
- For most suprasellar nonadenomatous pathologies, I remove only the anterior half of the rostrum; this minimal bone removal protects the pituitary gland and facilitates watertight skull base reconstruction at the end of tumor resection.
For additional illustrations of using endoscopes during skull base surgery, please refer to the Jackler Atlas by clicking on the image below:
References
Conger AR, Lucas J, Zada G, Schwartz TH, Cohen-Gadol AA. Endoscopic extended transsphenoidal resection of craniopharyngiomas: nuances of neurosurgical technique. Neurosurgical Focus. 37(4):E10, 2014.
Please login to post a comment.