Pituitary Adenoma: Diagnosis and Operative Considerations
Pituitary tumors were among the first tumors explored by the pioneer neurosurgeons. Their surgical history defines the history of our profession.
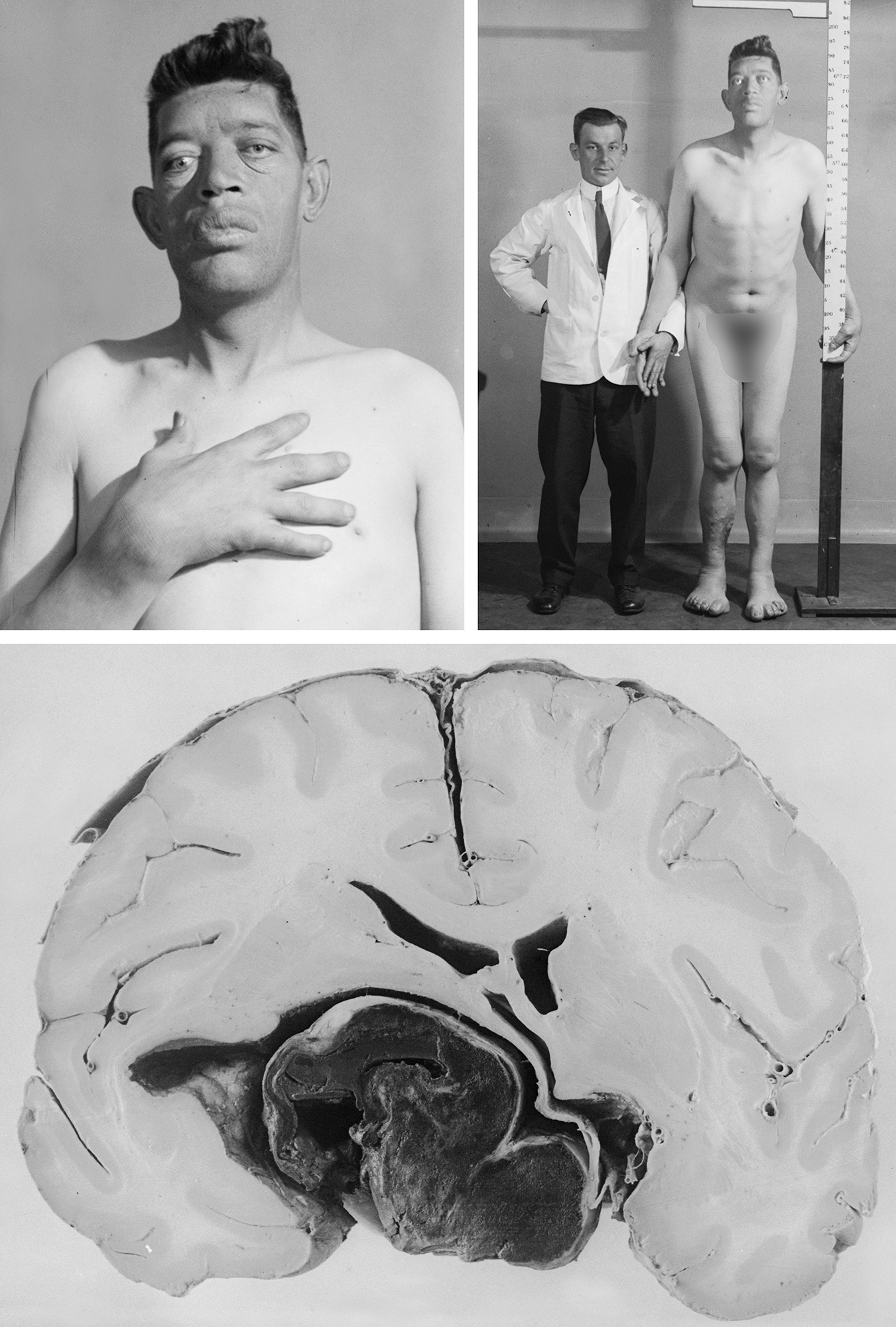
Figure 1: This patient was arguably one of Harvey Cushing’s most favorite patients. Cushing evaluated this patient in 1904 for acromegaly and operated on him through the transsphenoidal approach. The patient died immediately after his second operation. Near the turn of the century, transsphenoidal surgery literally placed Cushing on the brink of operating on the brain during the infancy of our specialty.
There are two main transsphenoidal approaches to expose pituitary tumors: endoscopic and microscope-assisted approaches. Transcranial approaches are exceedingly rarely necessary considering the reach of the endoscopic techniques. I have used the transcranial route only twice in my series of more than 400 pituitary tumor surgeries.
The advantages of the endoscopic versus the microscope-assisted route are numerous. The endoscopic method offers significantly enhanced direct visualization of the operative space and greater working angles, thereby minimizing the risk of an inadvertent injury to the surrounding structures. Specifically, macroadenoma resection is more efficient under endoscopic guidance because of the importance of wide visualization that increases the operator’s confidence in more aggressive maneuvers and resection. Microadenoma surgery is likely to have similar outcomes if microscopic or endoscopic assistance is employed.
The microscope-assisted approach facilitates the surgeon’s comfort because all neurosurgeons are familiar with its use. This technique implements a nasal speculum and provides fixed and narrow working angles toward the sella. However, the nasal speculum protects the nasal mucosa.
The disadvantage of microscope assistance is that visualization of the three-dimensional environment in the nasal compartments and intrasellar resection cavity is limited. Ring curettes are used to explore and “feel” the lateral portions of the intrasellar space to guide resection of the tumor (angled endoscopes expand the viewing angles). This “feel” requires significant experience and skill on the part of the surgeon to successfully perform a thorough resection.
The major indications for the transcranial approaches include the presence of intracranial invasion well beyond the boundaries of the sella. This approach may also be beneficial for tumors of unknown composition. The major limitations of a transcranial approach are the need for brain manipulation and the difficulty accessing the intrasellar component of the tumor.
Diagnosis
Presentations of patient with pituitary adenomas are diverse and largely depend on the nature of the cells composing the tumor. Presentation is commonly provoked by a hyperfunctional or hypofunctional endocrine syndrome, symptoms of mass effect such as headache or bitemporal hemianopsia, and/or the clinical syndrome associated with pituitary apoplexy. Patients with nonfunctioning adenomas commonly present with symptoms of mass effect on the optic chiasm.
Based on the association between pituitary adenomas and multiple endocrine neoplasia (MEN)-1 syndrome, preoperative evaluation should involve acquisition of a family history and potentially a serum calcium level test. This step in evaluation is helpful for identifying other MEN tumors if the patient has a strong family history.
Prolactin-Secreting Pituitary Adenoma
Prolactinomas represent 30% of pituitary adenomas and are therefore the most common cause of an anterior pituitary tumor. The unique presentation of this tumor in women involves menstrual abnormalities such as secondary amenorrhea, oligomenorrhea, delayed menarche, primary amenorrhea, or infertility associated with eumenorrhea. The existence of galactorrhea is less reliable, reported in ~30% to 80% of all patients.
Additional presenting symptoms include decreased libido, dyspareunia, headache, and psychological manifestations that may include hostility, depression, and anxiety. Pathologic analysis suggests that prolactinomas all originate from microprolactinomas, but not all microprolactinomas are bound to become macroprolactinomas over time.
Serum prolactin evaluation is a key feature of diagnosis for a prolactinoma. The normal serum prolactin level in a nonlactating and nonpregnant woman is less than 20ng/ml. Levels elevated above 20, but not markedly elevated (>200ng/ml), necessitate vigilance in clinical evaluation because hyperprolactinemia is frequently seen with macroadenomas (“stalk effect”) that are not actively secreting prolactin.
The mass effect of the tumor on the stalk induces suppression of dopaminergic input for inhibition of prolactin secretion. To definitely diagnose a prolactinoma, it is also necessary to rule out additional comorbidities such as hypothyroidism and systemic disorders. The use of dopaminergic antagonists, such as chlorpromazine, haloperidol, and metoclopramide should also be ruled out to definitely diagnose a prolactinoma.
The surgeon must also be vigilant for serum prolactin levels greater than 1,000ng/ml, as this is commonly associated with an invasive prolactinoma. The correct diagnosis of a true prolactinoma is important because it calls for the use of proven medical management with bromocriptine, pergolide mesylate, lisuride, cabergoline, or quinagolide for regression of the tumor. This is the only pituitary tumor that requires medical management as the primary mode of therapy.
The correct identification of tumor type is necessary when planning for surgical or medical intervention, especially if the tumor is suspected to be invasive. More extensive discussion of surgical management of prolactinomas is included in the chapter on pituitary microadenoma.
Growth Hormone-Secreting Pituitary Adenomas
Patients with growth hormone (GH)-secreting adenomas typically present with a syndrome encompassing adenoma mass effect and systemic signs of GH excess. The more common presentation of GH excess is acromegaly, in which excess is present after epiphyseal closure, as opposed to the pre-epiphyseal closure manifestation of gigantism.
Acromegaly encompasses generalized connective tissue, cardiovascular, and respiratory abnormalities, in addition to new-onset glucose intolerance. The most prominent physical features with this disease are coarse facial features, including prominent lips, nasolabial folds, and tongue and frontal scalp furrows. Features that are difficult to overlook are prognathism, increased spacing between the teeth, and frontal bossing. Signs that are less readily apparent include visceromegaly. This disease is often identified after an indolent course, with a mean time from onset to diagnosis of 8.7 years.
Endocrine criteria for GH-producing adenomas include a basal GH level of >5ng/ml and a lack of GH suppression (>2ng/ml) with an oral glucose tolerance test. There is also an elevation in serum IGF-1 levels secondary to the excessive serum GH. Importantly, 33% of patients with the disease exhibit an elevated serum prolactin level related to the previously described stalk effect. However, in some patients, this elevated prolactin level is a result of a plurihormonal somatotroph adenoma.
Medication therapies are an option for treatment of the acromegalic symptoms associated with elevated GH levels. The three groups of these medications used include somatostatin analogs, dopamine agonists, and GH receptor blockers. Definitive therapy includes surgical resection of the tumor.
Adrenocorticotrophic Hormone-Secreting Pituitary Adenomas
Adenomas that secrete excess adrenocorticotrophic hormone (ACTH), thereby producing secondary hypercortisolemia, result in the clinical syndrome known as Cushing’s disease. This diagnosis must be distinguished from Cushing’s syndrome, which is the generalized term for either an iatrogenic or pathologic excess of glucocorticoids. Ectopic ACTH-producing tumors, such as small-cell lung cancer, bronchial carcinoid, foregut carcinoid, medullary carcinoma of the thyroid, islet cell tumors of the pancreas, pheochromocytoma, and specific ovarian tumors, can also lead to Cushing’s syndrome.
Cushing’s syndrome is also observed in patients with corticotropin-releasing hormone (CRH)-hypersecreting tumors such as small-cell lung cancer, nephroblastoma, medullary carcinoma of the thyroid, prostatic carcinoma, colonic carcinoma, and bronchial carcinoid. Non-neoplastic conditions that are associated with low-level glucocorticoid excess are also considered for this differential, including obesity, alcoholism, iatrogenic glucocorticoid ingestion, depression, and chronic exposure to a stressful stimulus.
Cushing’s disease is clinically indistinguishable from Cushing’s syndrome. The patients’ features have a prominent centripetal fat deposition (primarily in the face, supraclavicular, and dorsocervical fat pads) and associated weight gain. The characteristic fat distribution in the face and dorsocervical fat pad is commonly referred to as a moon face and buffalo hump, respectively. There is also preferential fat deposition in the abdomen, referred to as truncal obesity.
The excess cortisol with this condition also impacts the integrity of the skin, causing atrophy of the epidermis and dermal layers. The impaired collagen synthesis and increased abdominal fat distribution generate purple striae. Hypercortisolemia also impairs the integrity of the dermal capillaries, predisposing the patient to easy bruising. Hirsutism is common. The physiologic consequences of Cushing’s syndrome include impaired glucose tolerance, hypertension, and osteoporosis. Skeletal manifestations include compression fractures and pathologic fractures of the pelvis, feet, or ribs.
Diagnosis of a corticotrophic adenoma must include verification of hypercortisolemia, a distinction between hypercortisolemia secondary to ACTH-dependent versus ACTH-independent etiologies, and lastly, confirmation that the pituitary is the source of ACTH versus an ectopic source.
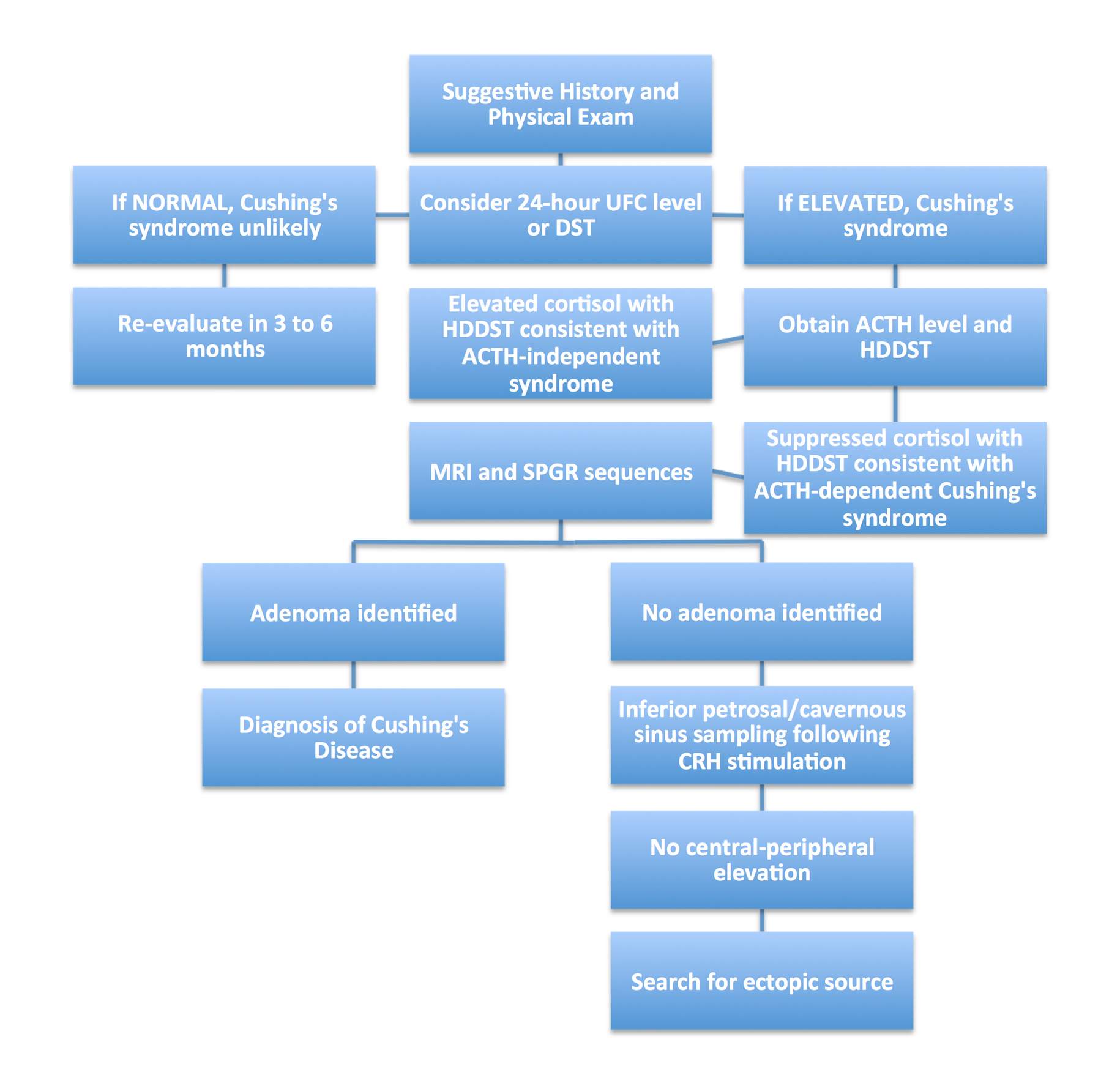
The verification of hypercortisolism compatible with Cushing’s disease is through urine-free cortisol levels, dexamethasone-suppression testing, and documentation of elevated serum ACTH levels. There should not be any cortisol suppression shown with low-dose dexamethasone tests, but there should be with high-dose dexamethasone tests. These tests will verify the correct diagnosis of Cushing’s disease.
If initial tests are not conclusive, additional studies may include bilateral inferior petrosal sinus sampling for confirmation of hyper-ACTH levels originating from the pituitary. The central-to-peripheral ACTH gradient ratio should exceed 2; a finding less than 1.7 is consistent with an ectopic source of ACTH. Surgical management is the standard of care for corticotrophic adenomas. Resection methods are discussed in the Pituitary Microadenoma chapter.
Figure 2: Decision tree for the diagnosis of Cushing’s disease. Abbreviations: UFC, urinary free cortisol; DST, dexamethasone suppression test; HDDST, high-dose dexamethasone suppression test; SPGR, spoiled gradient recall; CRH, corticotropin-releasing hormone.
Thyrotropin-Secreting Pituitary Adenomas
Pituitary adenomas that secrete excess thyroid-stimulating hormone (TSH) are rare, representing less than 1% of all pituitary adenomas and they are the rarest of the hyperfunctioning pituitary adenomas. The clinical manifestations of this syndrome are hyperthyroidism and diffuse thyroid enlargement secondary to excess TSH stimulation, precipitating formation of a goiter. These tumors infrequently have been documented to secrete GH and present with acromegalic features, in addition to thyrotropic symptoms.
Diagnosis of TSH-secreting adenoma requires serum analysis of TSH and thyroid hormones. For a positive diagnosis, the TSH should be elevated along with high thyroid hormone levels.
Therapy for these patients encompasses multiple modalities. Surgical resection, radiotherapy, and medical management can be incorporated as part of their multidisciplinary care. The standard of care is surgical resection; alternatively, use of somatostatin analogs has been advocated. The infrequent incidence of these tumors makes diagnosis difficult. A thyroidectomy is pursued in select cases. These tumors can present as invasive macroadenomas.
Evaluation
The primary focus of evaluation revolves around radiographic and endocrine diagnostic workups. These studies permit optimization of the surgical approach, perioperative patient management, and intraoperative anesthetic control.
The imaging modality of choice for evaluation of a pituitary mass is contrast-enhanced high-resolution MR imaging (3 Tesla) through the sella. High-resolution MRI is important in preoperative planning, defining the dimensions of adenomas larger than 3 mm (macroadenomas defined as >10 mm) and for identifying the boundaries of normal pituitary, subarachnoid cisterns, and parasellar contents. The acquisition of a thin-cut CT provides valuable information regarding the bony morphology of the sella and pneumatization of the sphenoid sinus.
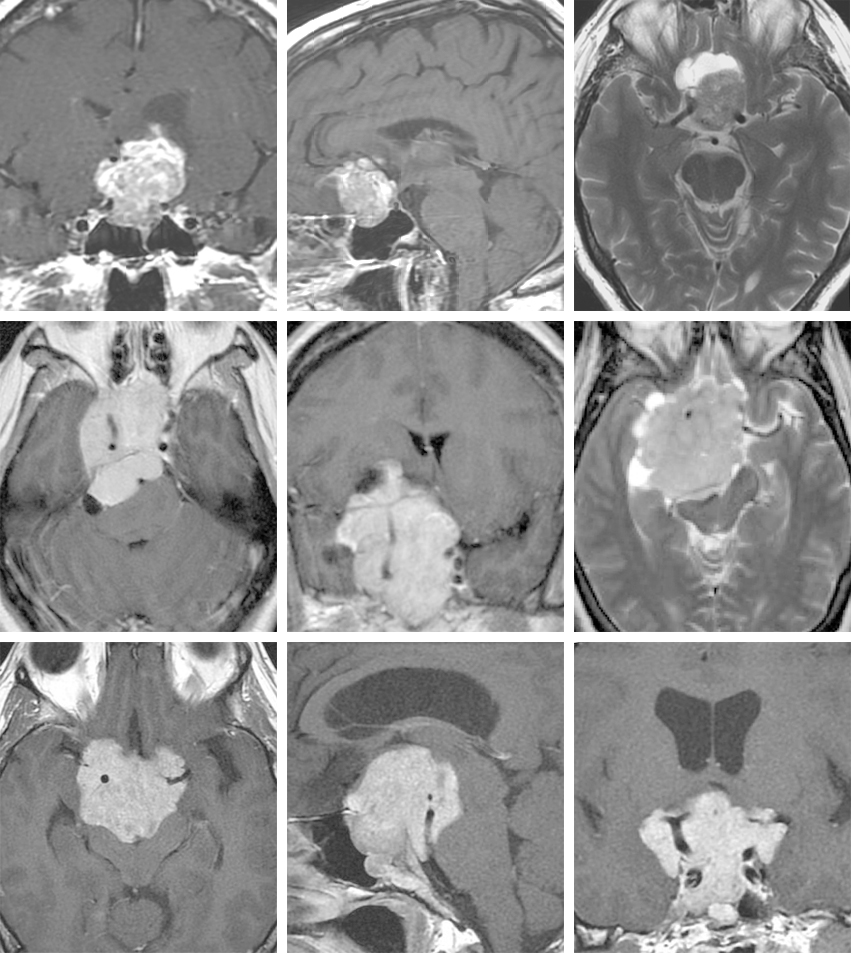
Figure 3: A pituitary microadenoma causing acromegaly is demonstrated on coronal and sagittal sequences. The tumor is hypointense compared to the pituitary gland.
Figure 4: Pituitary macroadenomas typically presents with optic apparatus compression (top images) and can reach a giant size (bottom images).
The prevalence of adenoma-associated endocrinopathies and implications in anesthetic management necessitate a complete preoperative endocrine evaluation. For example, Cushing’s disease is associated with comorbidities such as coronary artery disease, congestive heart failure, cardiomyopathy, hypertension, diabetes mellitus, and primary thyroid disorders.
To optimize the patient’s preoperative status, it is important to consider a referral to an endocrinologist or cardiologist for further evaluation and management. For definitive diagnosis of a suspected endocrinopathy and hyperfunctional adenoma, the patient may require specific laboratory studies (Figure 2). An endocrine evaluation can also provide a record of hypopituitarism to facilitate preoperative and postoperative hormonal comparison.
Some reports have described reversal of preoperative hypopituitarism, which has been attributed to surgical decompression. Therefore, in some cases, hypopituitarism may be related to disruption of the hypophyseal portal blood flow in the absence of glandular necrosis, and there may be an opportunity for reversal of this condition. A more detailed description of the endocrine diagnostic workup is mentioned in the above Diagnosis section for specific adenoma subtypes.
Patients who suffer from hypothyroidism and receive replacement before surgery should also receive cortisol replacement to prevent severe adrenal crisis. Careful attention to this detail can minimize the risk of a life-threatening crisis.
| Type of Adenoma | Test |
| Prolactinoma | Prolactin |
|
Chlorpromazine level |
|
| TSH | |
| Somatotrophic adenoma (Acromegaly) | GH suppression test |
| GH | |
| TSH | |
| Insulin-like growth factor I | |
| GHRH | |
| Corticotrophic adenoma (Cushing’s disease) | Dexamethasone suppression test (low/high dose) |
| Urinary free cortisol | |
| ACTH (peripheral or petrosal sinus sampling) | |
| Metyrapone stimulation test |
Table 1. Tests for endocrine evaluation are summarized. Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone; GHRH, growth-hormone-releasing hormone; TSH, thyroid-stimulating hormone
Indications for Surgery
Surgical indication for patients with a pituitary adenoma depends on the tumor type, size, and its functional status. The operative indications include symptomatic pituitary apoplexy and mass effect on the chiasm, Cushing’s disease, acromegaly, secondary hyperthyroidism, and failure of or intolerance to medical management.
Pituitary apoplexy refers to hemorrhagic infarction of a preexisting pituitary adenoma and indicates a need for urgent surgical intervention if visual deficits are present. There may also be limited extracapsular subarachnoid extension of hemorrhage. Patients with apoplexy may present with acute vision change, ophthalmoplegia, acute headache, meningismus, and/or adrenal crisis. Glucocorticoid replacement will prevent acute adrenal insufficiency. Prompt recognition of this syndrome is critical. Patients with intact visual fields, even if they have symptoms of ophthalmoplegia, may be treated conservatively.
Identification of a macroadenoma with chiasmal mass effect and documented bitemporal hemianopsia is a clear indication for surgical decompression. Prolactinomas are an exception to this rule because medical management with dopaminergic agonists can effectively shrink the adenoma and relieve the symptomatic mass effect. Intolerance or irresponsiveness to pharmacotherapy signifies the need for surgical resection.
It is imperative to avoid unnecessary surgical intervention on prolactinomas that mimic other operative tumors.
Figure 5: Prolactinomas may present with very atypical imaging features and mimic non-adenomatous tumors. In each row of the above images, a very atypical prolactinoma is diagnosed based on laboratory studies, obviating a need for surgical intervention. These atypical features demand preoperative endocrinological evaluation for most parasellar tumors to avoid unnecessary surgery. The sella is not significantly enlarged in the first row. Note the off-midline and asymmetric morphology of the second row images. The images in the third row mimic a very aggressive tumor.
Furthermore, cystic prolactinomas may not respond as well to medical therapy and they require cyst decompression. Patients with tumors that cause skull base erosion may undergo surgery because tumor regression by medical treatment may induce CSF leakage.
Contraindications to transsphenoidal surgery are infrequent, but they include active sinus infection and anomalous carotid arterial anatomy that impedes the transsphenoidal route. Anesthetic management in patients with endocrinopathies such as Cushing’s disease, acromegaly, or secondary hyperthyroidism can also contraindicate or, most likely, complicate surgical intervention. Preoperative medical management can often normalize these patients’ operative risk.
I tend to treat patients with nonsymptomatic nonfunctional tumors conservatively, following their tumors with serial imaging and visual field exams.
Metastatic tumors to the pituitary gland as the first presentation of the systemic disease are exceedingly rare. Their distinct presentation involves diabetes insipidus (DI).
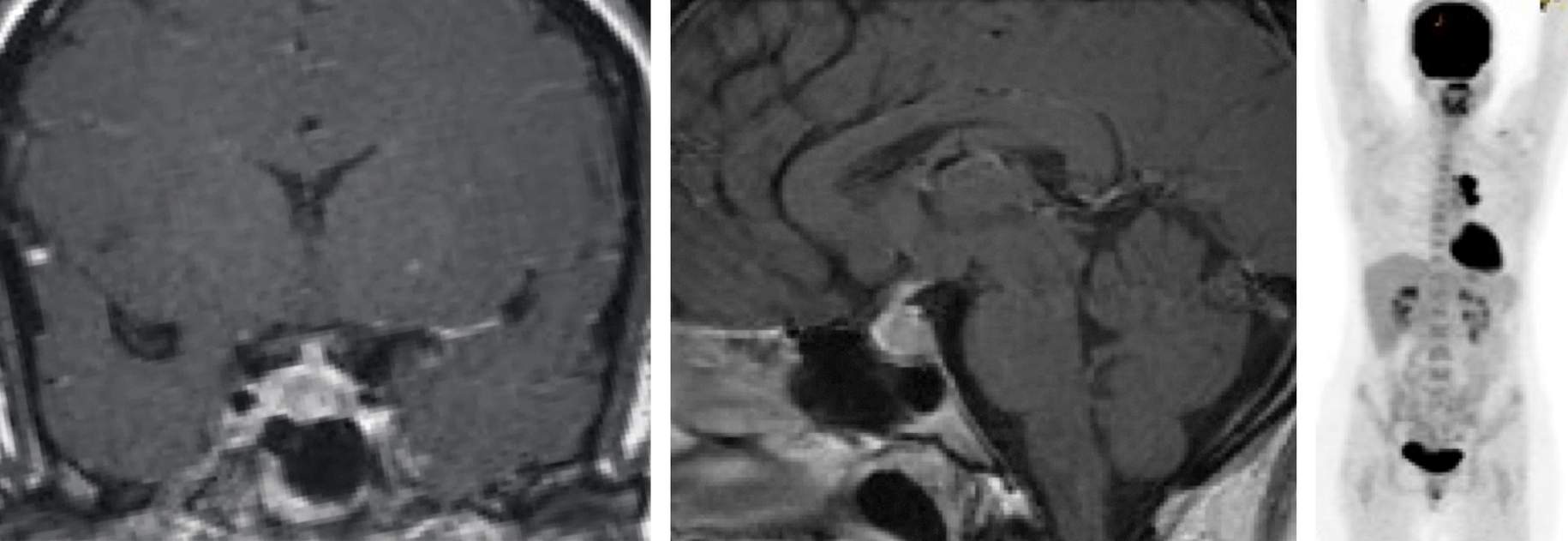
Figure 6: Unlike pituitary adenomas, metastatic tumors to the pituitary gland infiltrate the posterior pituitary and present commonly with diabetes insipidus (left two images). In this patient, a primary tumor of the lung was found on systemic imaging (right image). Noncompressive metastatic tumors to the pituitary are not amenable to resection and most likely should undergo radiation therapy.
Preoperative Considerations
Endocrinopathy and its consequences complicate anesthesia management. The presence of preoperative hypopituitarism requires the patient to be on steroid replacement therapy, and “stress doses” are indicated during the perioperative period. In patients with nonpathologic adrenal function, hydrocortisone dosing is a reasonable treatment option.
If secondary hypothyroidism is identified, it should be corrected preoperatively to optimize the patient’s stress tolerance. Patient’s suffering from Cushing’s disease require comprehensive preoperative medical evaluation to optimize their cardiopulmonary status. Patients with acromegaly require special preparations for their endotracheal intubation.
Careful study of the patient’s preoperative imaging is pertinent to accurately delineate the pituitary tumor from any sellar or parasellar vascular anomalies. The existence of aberrant arterial anatomy not identified preoperatively can predispose the patient to intraoperative arterial injury. This scenario is especially important with recurrent or invasive tumors that significantly alter the normal anatomy of the sella.
Intraoperative Considerations
The use of intraoperative CT navigation to guide the extent of bony removal along the floor of the sella is beneficial. The most common cause of subtotal tumor removal is inadequate bony exposure.
Bleeding from the midline dural venous plexus can be managed by cauterizing the dura during its opening. However, bleeding from the lateral margins of the surgical field is more likely from the cavernous sinus and requires thrombin-soaked Gelfoam packing and gentle tamponade. When bleeding originates from the venous sinus, even when minor, there is a concern for air embolism. Elevation of the patient’s head often helps decrease venous bleeding from the cavernous sinus, but excessive head elevation leads to a dramatic increase in the risk of air embolism.
In a patient with a macroadenoma, the native functional glandular tissue is frequently compressed against the sella posterosuperiorly. The distinction between the normal versus neoplastic glandular tissue can be difficult. The surface of the firm neoplastic tissue appears red-orange variegated with the vascular lattice. The difference in texture and integrity is an additional distinguishing feature; the neoplastic tissue is friable and can often be removed by suction or curettage.
Hemorrhagic complications of transsphenoidal surgery account for a noteworthy proportion of the morbidities associated with this operation. Common locations of hemorrhage include the epidural space, subarachnoid space, brain parenchyma, and sella, complicated by secondary intracranial extension. Extrasellar extension into the thalamus is also seen with larger tumors. The predominance of hemorrhages is associated with suprasellar instrumentation or persistent bleeding within the resection cavity at the end of resection. The most common cause of disappointing postoperative hemorrhage is subtotal resection of the tumor. This is particularly likely in older patients previously consuming aspirin.
The operator should avoid blind instrumentation-induced injury to the circle of Willis branches. Such maneuvers invariably lead to subarachnoid hemorrhage and vasospasm. A major sign of such an injury is the occurrence of early postoperative decline in visual acuity. Radical tumor removal and sufficient hemostasis are paramount. The risk of vasospasm after resection of giant tumors even in the absence of subarachnoid hemorrhage is significant and should not be overlooked. A sufficient index of suspicion is necessary.
Carotid Artery Injury
Intracavernous carotid artery injury is a rare but serious complication. With this complication, torrential bleeding is encountered during instrumentation of the lateral sella and intracavernous space that results in injury to the carotid artery wall or avulsion of its arterial branches. Persistent trigeminal artery, abnormal carotid anastomoses, and internal carotid aneurysm can be causes of arterial injury/torrential bleeding and should be recognized preoperatively.
If massive arterial bleeding occurs during transsphenoidal surgery, immediate packing with cottonoid patties is necessary to achieve hemostasis. However, forceful packing can potentially enlarge the defect in the wall of the immobile carotid artery. Secondary complications of intracavernous arterial injury include vasospasm, false aneurysms, carotid cavernous fistulas, and carotid occlusion. Therefore, upon intraoperative packing and hemostasis, the procedure should be abandoned and an arteriogram obtained to exclude these secondary complications.
Transsphenoidal Surgery: Carotid Artery Injury
Other Considerations
Postoperative vision loss is a rare complication, but devastating. The etiology of this complication can be from operative trauma, chiasm prolapse or ischemia, mass effect of a suprasellar hematoma, or aggressive packing to achieve intraoperative hemostasis. Major risk factors for postoperative vision loss include large or recurrent tumors, dumbbell-shaped tumor morphology, preoperative visual impairment, and surgeon’s inadequate experience.
Dumbbell-shaped anatomic configuration of a tumor is caused by constriction imposed on the tumor by the diaphragm. This configuration makes a complete removal of the suprasellar segment difficult through the transsphenoidal approach. The operator may be tempted to use techniques such as blind suctioning or curettage. These maneuvers should be strictly avoided. The limitations of transsphenoidal surgery should be recognized for its safe and effective application.
Other technical factors can increase the risk of visual compromise. Aggressive opening of the self-retaining speculum once present in the sphenoid sinus can induce optic canal fracture and optic nerve injury.
Closure
The details of closure are dictated by the presence or size of the defect in the diaphragm. Multiple materials have been used to pack the sella, including autologous adipose tissue, oxidized cellulose, or Gelfoam. I use small pieces of adipose tissue wrapped in Surgicel. This composite allows me to handle the fat globules more effectively and direct or deposit them near the site of the defect. In the absence of an obvious leak, I gently pack the sella with fat and reconstruct the sellar floor with a small sheet of prosthesis.
Figure 7: Routine sellar packing after transsphenoidal surgery often calls for autologous adipose tissue wrapped in Surgicel. A piece of prosthesis is countersunk against the bony edges to keep the packing in place. I believe the most common reason for postoperative CSF leakage is displacement of packing materials because of a lack of rigid construct at the floor of the sella to keep the fat in place.
Figure 8: A tear in the diaphragm and resultant CSF leakage often occurs during blind removal of tumor located along the anterior aspect of the resection cavity above the tuberclum sella (anterior sellar recess), where the diaphragm is attached anteriorly (see inset image). It is important to identify the diaphragm early during surgery and maintain its integrity throughout resection. If an inadvertent leak is apparent, I place and pack the fat at the exact location of the leak and avoid indiscriminately and forcefully packing the entire sella because this can lead to suprasellar mass effect and visual worsening.
Errors during the repair relate to migration of the packing material, overpacking the intrasellar space, and intracavitary expansion of certain packing materials, such as Gelfoam.
Prevention of delayed nasal deformity is an important consideration during the initial approach and closure. The nasal cartilage should be maximally preserved during exposure. Following reconstruction of the sella, the speculum is removed and in an attempt to avoid major septal deformity or nasal passage obstruction, the septal cartilage is manually repositioned to the midline, adjoining the bony nasal spine.
Up to 8% of patients who undergo this surgery experience perforation of the cartilaginous septum related to mucoperichondrial damage secondary to surgical instrumentation or postoperative infection. This complication can lead to crusting, pain, whistling, and scarring-induced nasal passage obstruction. In an attempt to reestablish the mucosal-septal interface, the nares should be packed at the end of the operation.
Postoperative Considerations
Standard postoperative precautions are recommended for these patients, including avoidance of nose-blowing and unnecessary bearing down. Hydrocortisone is administered postoperatively and is tapered at a rate that accommodates the patient’s endocrine functional status.
Postoperative cerebrospinal fluid (CSF) rhinorrhea is a more frequent complication after transsphenoidal surgery with a prevalence of approximately 3%. There are four major identified factors that are predictive of postoperative CSF leakage:
- tumor size (>10 mm),
- previous resection or radiation therapy,
- preoperative CSF leakage, and
- intraoperative CSF leakage.
As mentioned previously, the anterior recess of the suprasellar cistern is often the source of CSF leakage if the surgeon unintentionally and blindly dissects the adherent tumor or opens the dura too far superiorly. Recurrent tumors are notorious for CSF leaks.
Regardless of pristine surgical technique and preservation of the diaphragm, occult CSF leakage can occur. I have adopted the practice of inducing multiple Valsalva maneuvers before completing the closure. The integrity of the surgical closure is an important factor in the prevention of CSF rhinorrhea.
If a CSF leak is apparent postoperatively, I install a lumbar drain for 3-4 days. If ineffective, I proceed with a re-exploratory surgery and repack the sella and potentially the sphenoid sinus. Communicating hydrocephalus is an infrequent cause of sustained rhinorrhea, which can be treated through placement of a shunt.
Sinusitis is also a potential complication of transsphenoidal surgery, which occurs secondary to inflammation and obstruction of the paranasal sinus drainage pathways. This complication is typically mild and self-limiting. Consideration may be given to prescribing a course of antibiotics and decongestant therapy to relieve blockage and facilitate sinus drainage.
Postoperative Diabetes Insipidus
Diabetes insipidus (DI) is one of the more frequent postoperative complications after transsphenoidal surgery. The etiology of DI relates to distention or injury of the neurohypophysis or pituitary stalk. Careful monitoring of fluid input/output, urinary osmolality, and serum sodium are critical during the immediate postoperative period because serum sodium may rapidly escalate to dangerous levels (>145mEq/L).
Routine early postoperative diuresis related to intraoperative fluid boluses must be distinguished from DI. This can be achieved with a water deprivation test; early postoperative diuresis related to intravenous fluids will not impact plasma osmolality or sodium. The water deprivation test involves withholding water intake for 6 to 8 hours and checking urine osmolality, which in the setting of DI fails to exceed 200mOsm/kg because of a deficiency in the patient’s ability to concentrate the urine. This will correspond to a normal response in plasma osmolality, such that it can approach 320 to 330 mOsm/kg.
Treatment for DI depends on the patient’s functional status. A conscious patient with an intact natural thirst mechanism can maintain plasma osmolality through being instructed to drink only when thirsty. Patients with severe DI or those who are unconscious can be managed with desmopressin (DDAVP). Approximately 60% of patients experience transient DI postoperatively, with less than 3% suffering from permanent DI. Most patients who have undergone uncomplicated transsphenoidal surgery can be managed with a single dose of desmopressin postoperatively.
Postoperative hypopituitarism is often a result of destruction of nonpathologic adenohypophyseal tissue. This hypopituitarism ranges in severity and duration, and there is a possibility of complete and permanent impairment. Women who plan to become pregnant should be advised regarding this risk.
Postoperative Evaluation of Functional Tumors and their Remission
The success of resecting an ACTH-secreting microadenoma can be evident on laboratory analysis between postoperative days 2 and 3. Standard laboratory evidence suggestive of complete resection includes an undetectable serum ACTH level and morning cortisol <5mcg/dl. Importantly, if the serum cortisol level decreases dramatically postoperatively, but remains within the normal physiologic range, it generally indicates an incomplete resection.
For these patients, a re-exploratory transsphenoidal operation is reasonable if the diagnosis of Cushing’s disease is biochemically unquestionable. Other options for further therapy include medical and radiation therapy, as well as bilateral adrenalectomy.
Patients suffering from Cushing’s disease should be monitored in the intensive care unit postoperatively so that occurrence of an acute syndrome of hypocortisolism can be quickly diagnosed and treated. This diagnosis is a desirable finding because it almost always signifies biochemical cure.
Remission from acromegaly has been defined as an postoperative serum IGF-1 level within normal limits and a GH <1ng/ml drawn during an oral glucose tolerance test (OGTT). Historically, a basal GH <5ng/ml may indicate a surgical cure; however, this measure has been questioned. By meeting these remission criteria with an OGTT GH and IGF-1, the basal GH level is nearly universally <5nh/ml; however, this is not a reciprocal relationship. Therefore, examination for remission should include OGTT GH and IGF-1 measurements.
Pearls and Pitfalls
- Steps to reduce the risk of postoperative hemorrhage include 1) avoiding cavernous sinus entry, 2) gross total tumor resection, and 3) ensuring sufficient hemostasis within the resection cavity.
- Meningitis can be an initial sign of postoperative CSF rhinorrhea.
- Errors during sellar repair relate to migration of the packing material, overpacking the intrasellar space, and intracavitary expansion of certain packing materials.
Contributor: Benjamin K. Hendricks, MD
For additional illustrations of using endoscopes during skull base surgery, please refer to the Jackler Atlas by clicking on the image below:
References
Greenberg MS. Handbook of Neurosurgery. 7th ed. Canada: Thieme, 2010.
Jane JA, Thapar K, Laws ER. Pituitary tumors: Functioning and nonfunctioning, in Winn HR (ed): Youmans Neurological Surgery. 6th ed. Philadelphia: Elsevier Saunders, 2011, pp. 1476-1510.
Tindall GT, Woodard EJ, Barrow DL. Pituitary adenomas: General considerations, in: Apuzzo MLJ (ed). Brain Surgery. New York: Churchill Livingstone; 1993. pp. 269-276.
Please login to post a comment.