Techniques of Sylvian Fissure Split
This is a preview. Check to see if you have access to the full video. Check access
Step-by-Step Dissection of the Sylvian Fissure Using the Inside-to-Outside Technique
This is a preview. Check to see if you have access to the full video. Check access
Dissection of a Difficult Fissure for a Ruptured MCA Aneurysm
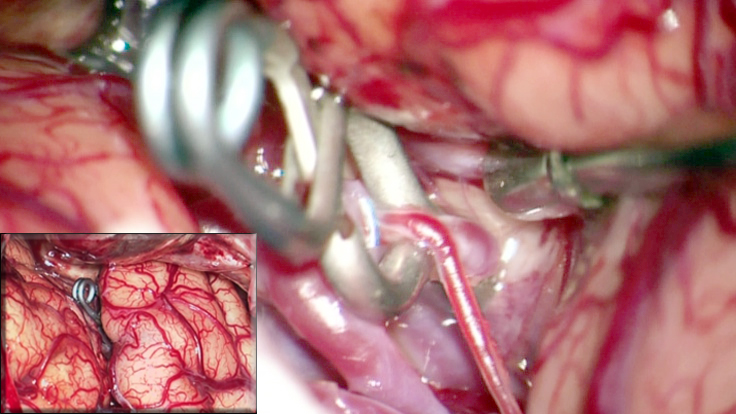
Figure 1: Generous dissection of the Sylvian fissure allows atraumatic clip ligation of cerebral aneurysms (inset) using dynamic retraction.
The evolution of microsurgical techniques has facilitated preservation of normal brain tissue while providing surgeons with a reasonable access to deep brain lesions. The application of these techniques to intra-cisternal and subarachnoidal dissection has created safe surgical corridors. Specifically, the dissection of the Sylvian fissure allows exposure of the structures around the anterior circle of Willis, parainsular regions, mesiobasal temporal lobe, and interpeduncular cisterns, with minimal invasion into or retraction of the normal brain parenchyma.
A safe and efficient “splitting of the Sylvian fissure” requires familiarity with the surgical anatomy of the structures surrounding the fissure and the fundamentals of microsurgical techniques. A wide opening of the Sylvian fissure is technically challenging; this task frequently does not receive the respect or attention it deserves. Mastery of efficient fissure dissection is imperative so that the operator does not arrive at the critical part of the operation partially fatigued.
In this chapter, I will review the surgical techniques applicable to exploration of the Sylvian fossa to gain access to the carotid-optic cistern and insula. There are certainly some “easy fissures” in older patients with brain atrophy, and “difficult fissures” in young patients with aneurysmal subarachnoid hemorrhage. Each scenario requires a different set of microsurgical skills and maneuvers for safe and efficient fissure dissection.
Indications for the Procedure
There is much controversy regarding how much of the fissure should be dissected to safely reach the anterior skull base or anterior circle of Willis.
Proponents of extensive fissure dissection believe that the disconnection of the frontal and temporal lobes will lead to more operative space with less basal frontal lobe retraction along the anterior skull base and basal cisterns. Some operators routinely dissect the fissure generously, whereas others do a minimal anterior fissure split.
I believe the extent of required Sylvian fissure dissection depends on the location and texture of the underlying pathology as well as the brain compliance (the extent of brain atrophy and absence of brain swelling,) and the skill of the operator in using dynamic retraction efficiently while avoiding fixed retraction. Essentially, the complex interaction between the degree of technical difficulty in managing the aneurysm or removing fibrous vascular tumors and brain resistance to mobilization determines the need for a generous Sylvian fissure split.
There are two extremes in the need for Sylvian fissure split. For large insular tumors or giant middle cerebral artery (MCA) aneurysms, the fissure must be dissected as widely as possible to the level of the superior and inferior peri-insular sulci. The posterior extent of dissection is determined by the location of the posterior pole of the tumor and the safety of dissection between very adherent posterior interdigitations of the fissure, especially in the dominant hemisphere.
For most patients who harbor anterior skull base tumors or anterior circulation aneurysms, I dissect only the anterior limb of the Sylvian fissure, exposing the cistern just anterior to the M1. If additional fissure split is deemed necessary during handling of the lesion, I redirect my attention to attempt more extensive split.
Overall, some degree of atraumatic fissure dissection is mandatory for the lateral subfrontal trajectory.
Microsurgical Operative Anatomy of the Sylvian Cistern
The Sylvian cistern contains three parts: the fissure, the opercular sulci, and the Sylvian fossa. The Sylvian fossa houses the MCA that is just superficial to the insula.
Figure 2: The Sylvian fossa contains the MCA and is the space just lateral to the insula (image courtesy of AL Rhoton, Jr).
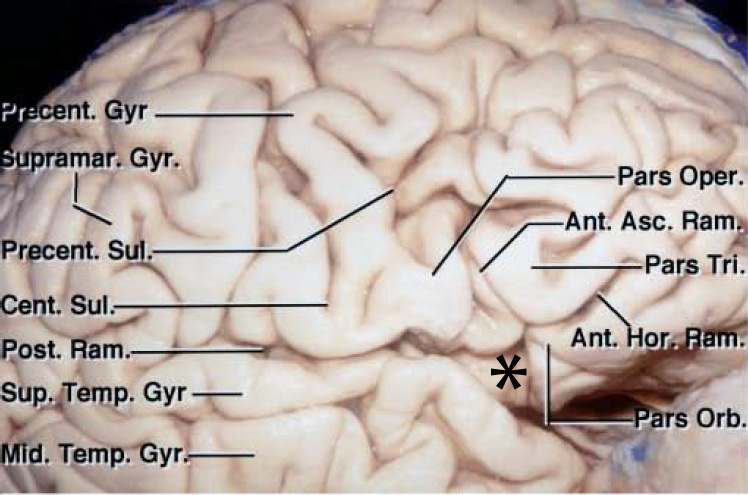
The fissure is divided into anterior (stem) and posterior (insulo-opercular) compartments. The stem originates inferiorly at the anterior perforated substance and extends laterally to the temporal pole. The stem reaches laterally and divides into the ascending, horizontal, and posterior rami; the confluence of these rami has been referred to as the “Sylvian point.”
Figure 3: The Sylvian point is marked with *. The horizontal and ascending rami divide the inferior frontal gyrus into the pars orbitalis, pars triangularis, and pars opercularis. The Sylvian fissure contains several interopercular sulci between the opercular surfaces of lateral orbital, inferior frontal, inferior parietal, and superior temporal gyri. These sulci are often oblique and curved due to opposition of the adjacent gyri. This configuration of the sulci makes fissure dissection a demanding task requiring the surgeon’s patience (image courtesy of AL Rhoton, Jr). The length of the fissure proximal to the Sylvian point is considered the “proximal” fissure and the corresponding length distal to this point is called the “distal fissure.” Most cases need a proximal Sylvian fissure dissection.
Figure 4: The proximal section of the fissure (vallecula) houses the internal carotid artery bifurcation and limen insula, where the MCA bifurcates into its superior and inferior trunks. The vallecula also contains lateral lenticulostriate perforators and the deep Sylvian vein. Opening of the vallecula will provide space to reach the proximal MCA and internal carotid artery bifurcation territories.
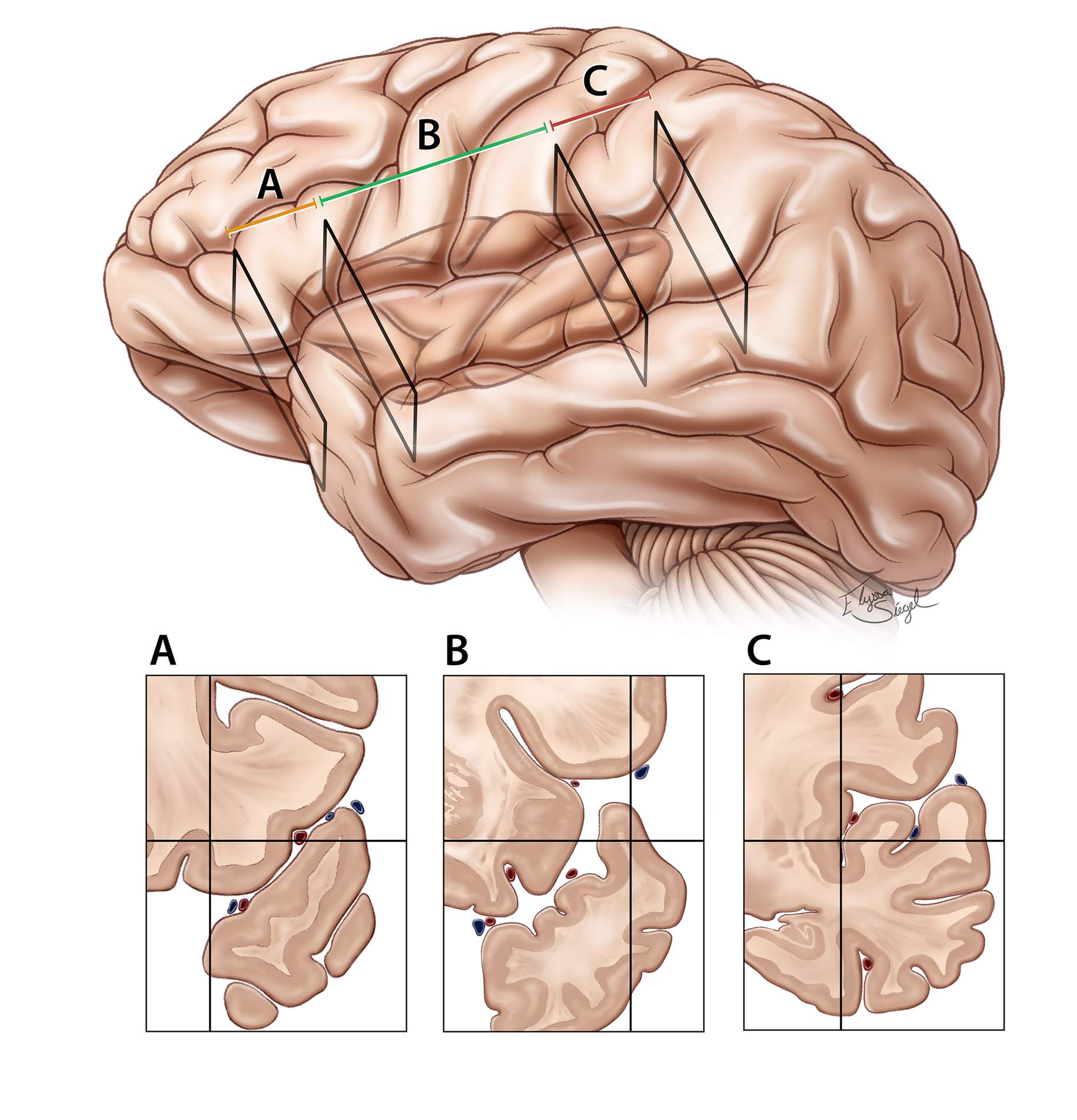
The proximal (sphenoidal) section (A) also includes the area (3-4 cm) over the planum polare where the pial surfaces can be highly adherent, requiring gentle microdissection. The paucity of the vessels in this section allows adherence of the frontotemporal opercula.
The middle (insular) section (B) is 6 to 7 cm in length and extends from the limen insula to the posterior insular point. In this section of the fissure, the sulci are less interdigitated, possibly simplifying fissure dissection.
The posterior (retroinsular) section (C) is short (4–5 cm) but deep, and covered by the supramarginal, transverse temporal, and transverse parietal gyri. The dissection can be especially challenging at this segment because of complex interdigitations of the opercula.
There is a dense network of pia-arachnoid fibers around the arteries, veins, and pial surfaces of the adjacent opercula and insular gyri of the entire fissure.
The MCA is divided in four segments: the M1 segment courses posterior and parallel to the sphenoid ridge, the M2 segment resides on the limen insula, the M3 segment spreads over the frontotemporoparietal opercula, and the M4 segment is composed of the branches to the cerebral convexity.
The course of the M1 segment (3–4 cm in length) within the proximal Sylvian fissure is C- or S-shaped. There are a number of anatomic variations in MCA branching, and familiarity with these anatomic variations is important for surgery of neighboring lesions. Please refer to the chapter on Clip Ligation of MCA Aneurysms for a review of these variations.
Adequate exploration of the MCA at the point of its origin from the internal carotid artery through its bifurcation will allow appropriate identification of the surgical vascular anatomy and preservation of normal structures during surgery in this region. The dissection should be conducted along the inferior and anterior aspect of the M1 to prevent inadvertent injury to the lenticulostriate arteries.
SYLVIAN FISSURE DISSECTION
The patient is usually positioned supine on the operating table with his or her head rotated away from the side of the surgery approximately 30 degrees. Turning the head more than 30 degrees may obstruct fissure dissection by rotating the temporal opercula into the angle of fissure dissection.
After the dural opening, further brain relaxation may be immediately achieved by gentle elevation of the anterior frontal lobe and opening the arachnoid membranes over the opticocarotid cisterns. A small cotton ball may be inserted underneath the frontal lobe to maintain the outflow of cerebrospinal fluid from these cisterns during fissure dissection.
I cover the surface of the brain, except the peri-insular areas, with pieces of moist Telfa to avoid heat injury to the cortex from the intense light of the microscope.
The Sylvian fissure is covered along its entire length with a thick band of arachnoid membrane. The superficial Sylvian veins outline the course of the fissure. In the absence of the veins, identification of the fissure may be difficult; in such cases, recognition of the M4 branches exiting the fissure onto the cortex may be helpful. The fissure is 10 to 14 cm in length, longer than often appreciated. Minor or subtle forms of cortical malformation can transform the fissure into a series of sulci, making the operator’s job very difficult.
Figure 5: The traditional Sylvian fissure opening involves splitting approximately the anterior one-third of the fissure (namely, Sylvian stem, proximal fissure: between the internal carotid artery bifurcation and pars triangularis) also known as the anterior limb, exposing the M1 and medial 2 cm of the M2 segments. The extent of this traditional split is demonstrated. The arrows define the neck of the posterior communicating artery aneurysm approached via the transsylvian route.
The Sylvian fissure is more readily split by conducting dissection above rather than below the superior Sylvian vein since the vein travels approximately 4 mm below the fissure in more than 80% of the hemispheres. The arachnoid of the fissure should preferably be opened on the frontal side of the veins, so that the veins will not cross the fissure when the frontal lobe in elevated.
If more than one superficial Sylvian vein is present, I prefer to dissect between the two veins because these veins are encased by arachnoid bands that are typically more robust than the pia. There is often no bridging vein between the superficial Sylvian veins, and therefore the small fronto-orbital tributary veins to the superficial Sylvian vein are less likely to be sacrificed during fissure opening. Preservation of the veins during fissure dissection protects cerebral venous drainage and voids venous infarction; this is especially important if these veins seem to be dominant based on their large caliber and parasagittal veins are less prominent on preoperative angiography.
The natural superior retraction of the apex of the pars triangularis along the Sylvian point typically provides the surgeon with the widest transfissure corridor where the superficial arachnoid membrane is demarcated; I resume the Sylvian fissure opening at this specific location.
Furthermore, exploration of the fissure at this point will expose the insular apex, an important landmark for surgical orientation. Using a round blade (beaver knife), I make a small (3-mm) opening along and above the superficial Sylvian vein. The superficial arachnoid membrane along the fissure may be incised at several points in a similar fashion. Next, I extend the opening through the arachnoid by holding the edges of the arachnoid with two short, fine bipolar or jeweler forceps, stripping the arachnoid over the vein and splitting the superficial fibers of the fissure.
If slight pial bleeding is encountered, hemostasis is achieved using a minute piece of gelfoam covered with a small cotton pledget, and bipolar coagulation is avoided during the entire fissure dissection, if possible. Exploration may be conducted a few millimeters further in the neighborhood of the oozing pial surface. The surgeon then may return to this region in a short period of time to appreciate the spontaneous hemostasis obtained using this technique.
Soft, moist, cotton pledgets (1Ï1 mm) or balls are gently glided between the pial membranes of the adjacent gyri. Gradual and gentle compression over the pledgets by the fine suction tube, in addition to the spread action of the bipolar forceps, will allow gradual extension of the initial opening down to the Sylvian fossa. A slightly larger pledget is introduced into the initial window to replace the smaller pledget as the fissure opening is enlarged and deepened. The larger pledget can keep this segment of the fissure open as dissection is continued anteriorly without the need for retractors.
I split the fissure deep enough to identify the MCA branches and insula in the Sylvian fossa at the site of the initial fissure opening. The dissection then proceeds in the anterograde fashion while opening the fissure from deep toward the surface or “inside-to-outside.”
Figure 6: The Sylvian fissure is split using the inside-to-outside technique. The dissection is started at the Sylvian point and extended to the depth of the fissure so that I can identify distal MCA branches on the surface of the insula. I then pursue dissection from deep to superficial (upper image, inset, arrow). This method of opening the fissure has an analogy. Yasargil compared this method to radial splitting of peeled orange wedges. It is difficult to separate the edges from the outside (left lower image), but it is easy to put my finger into the middle of the orange and radially separate the wedges (right lower image).
It is often more difficult to separate orange slices from outside the orange, but if one starts by entering into the center of the orange and identifying the dividing planes of the wedges from inside, one can split the slices more readily without compressing the individual slices and releasing their juice.
The inside-to-outside technique allows early identification of the MCA branches, therefore allowing the surgeon to adjust the plane of dissection along the interdigitating opercula while maintaining the depth of the fissure as a landmark for orientation.
The contours of the interdigitating opercula rarely follow a straight vertical line as the lateral orbital gyrus indents the proximal superior temporal gyrus and temporal pole, causing a C- or S- shaped course of proximal fissure in the coronal plane. Early identification of the undulating pial and arachnoid planes from inside the fissure simplifies dissection tremendously. As the fissure is opened toward the surface, the thicker superficial arachnoid membranes may be cut using microscissors.
Following identification of the MCA branches and insula deep at the Sylvian point, anterograde inside-to-outside dissection continues. Injection of saline solution using a syringe deep into and along the Sylvian fossa can expand this fossa, facilitating the identification of the arachnoid planes between the pial banks.
I like to use jeweler’s forceps with fine tips to grab and separate the more superficial thick arachnoid bands, when needed.
I continue deeper dissection using straight (nonbayoneted) microscissors and bipolar forceps (5-mm tips). Through the alternating use of bipolar forceps to spread the thin arachnoid layers, and microscissors to transect thick ones, the initial fissure opening is enlarged. Forceful blunt dissection or separation of the thick arachnoid bands or adherent pial banks using the spreading action of the forceps will lead to pial injury and bleeding. The use of bipolar coagulation to stop pial bleeding may actually lead to further cortical injury and further bleeding.
The planum polare or the flatter surface of the anterior fissure on the temporal side may be very adherent to the frontal lobe. Patient microsurgical dissection is warranted. Thick arachnoid layers cover the most anterior limb of the fissure just behind the sphenoid wing; sharp dissection in this area may require sacrifice of one of the superior Sylvian vein branches.
As the more proximal part of the fissure is split, the MCA bifurcation and the M1 branch is identified and a more medial dissection along this artery will allow expansion of the vallecula and creation of a surgical corridor toward the opticocarotid cisterns. The posterior frontal operculum often tends to herniate into the transsylvian corridor; its aggressive retraction leads to its venous congestion, and spontaneous cortical bleeding and must be avoided.
Along the medial aspect of the Sylvian fissure and just before reaching the opticocarotid cisterns anteriorly, a thick arachnoid band tethers the frontal and temporal lobes to each other. This band and its occasional encasing small vein are transected. A “T” shaped arachnoid incision is made over the opticocarotid cisterns with the anterior limb of the incision just lateral to the optic nerve and over the carotid artery. This incision is further extended medially to disconnect the posterior gyrus rectus from the chiasm. The posterior limb of the “T” incision can parallel the approximate course of the posterior communicating artery and connects with the arachnoid opening along the medial sylvian fissure.
The following steps summarize the principle maneuvers for Sylvian fissure dissection.
Step 1: The superficial fissure is dissected open at the level of the Sylvian point where the interopercular space through the arachnoid band is most prominent. This dissection is extended to the level of the M2 branches and insula. Note the use of the small cotton pledget discussed above.
Step 2: The very superficial thick arachnoid layer encasing the veins may be disrupted using fine forceps or microscissors. A microcotton ball is used to keep the dissection planes open and avoid direct contact between the suction tip and the pial surfaces.
Step 3: Next, I use the distal deep opening within the fissure and identify the distal MCA branches as a landmark to further open the fissure from inside-to-outside or deep-to-superficial in the posterior-to-anterior direction.
Step 4: The planum polare may be very adherent, and a round knife may be used to sharply identify the dissection and pial planes. Please note the roadmap for the inside-to-outside technique (inset image, green arrows).
I avoid the commonly used “outside-to-inside” technique, which is more difficult to perform because of the adherence of the frontotemporal opercula and the lack of any landmarks to guide dissection within the interdigitating opercula.
After extension and completion of Sylvian fissure dissection more medially along the distal M1 segment, the temporal operculum is mobilized away from the insula. The presence of MCA branches between the temporal operculum and the insula facilitates mobilization of the temporal operculum more than the frontal operculum. The superior and inferior peri-insular sulci can be identified, if necessary. The M1 branch may be used as a landmark to reach the opticocarotid cisterns and the internal carotid artery bifurcation.
Distal Fissure Dissection
Distal fissure dissection is often limited because of adherence of the posterior opercula at this level; aggressive manipulation in this area will place the superior temporal gyrus at risk of injury; this can be a factor especially in the dominant hemisphere. Dissection of the posterior fissures is necessary only for large insular tumors, M2/M3 aneurysms, and giant MCA bifurcation aneurysms.
A round arachnoid knife may be used to work within the adherent pial surfaces.
Gentle dynamic retraction of the frontotemporal opercula and anterior-to-posterior working angles often provide good exposure of the retrosylvian fossa, posterior insular cortex, and the peri-insular sulci, as well as the posterior M2 branches.
Pearls and Pitfalls
- I do not dissect the fissure from proximal to distal. This maneuver requires significant frontal lobe retraction as the M1 is identified early and followed toward its distal pathway. However, distal-to-proximal dissection does not provide early proximal control and poses certain risks, especially in the case of a ruptured aneurysm.
-
Repeated strokes on the pia by the shaft of the suction during dynamic retraction should be avoided. The suction should be “parked” at a strategic point for mobilization of each segment of the opercula and only moved if necessary. The tip of closed microscissors is used as a dissecting tool and the jaws for transection of the thick arachnoid bands.
-
The fissure is not perpendicular to the insula but is oblique as dissection proceeds in the posterior to anterior direction. One should not inadvertently violate the frontal pial banks while wandering within the fissure.
-
The use of dynamic retraction, sharp dissection and microscope’s mouthswitch through the inside-to-outside technique are fundamental ingredients for efficient fissure split.
Please login to post a comment.