Periventricular AVMs
This is a preview. Check to see if you have access to the full video. Check access
Posterior Callosal (Splenial) AVM
Please note the relevant information for patients suffering from arteriovenous malformations is presented in another chapter. Please click here for patient-related content.
Ventricular and periventricular arteriovenous malformations (AVMs) arise from the ependymal surface of the ventricular system and involve the choroid plexuses, as well as the subcortical structures that form the walls of the ventricular system.
Resection of ventricular lesions is different from resection of parenchymal AVMs because less perinidal dissection is necessary. This lack of parenchymal support or buttress is theorized to predispose these types of AVMs to a higher risk of hemorrhage, and therefore a more aggressive surgical philosophy may be justified.
A thorough understanding of the anatomic structures and landmarks within and around the ventricles is crucial for safe and complete resection of these complex lesions. Control of the deep white matter feeders poses a special challenge because the perinidal neural tissue is eloquent. The working depth is long and the operative window is narrow; these factors add to the technical complexity of AVM resection.
Operative Anatomy
The ventricular system is composed of the lateral, third, and fourth ventricles. Lateral ventricles irrigate into the third ventricle through their respective foramina of Monro. The third ventricle connects to the fourth ventricle via the aqueduct of Sylvius. The fourth ventricle subsequently drains into the cerebellomedullary cisterns via the bilateral foramina of Luschka and the midline foramen of Magendie. For more details, please refer to the Anatomy of the Ventricular System chapter.
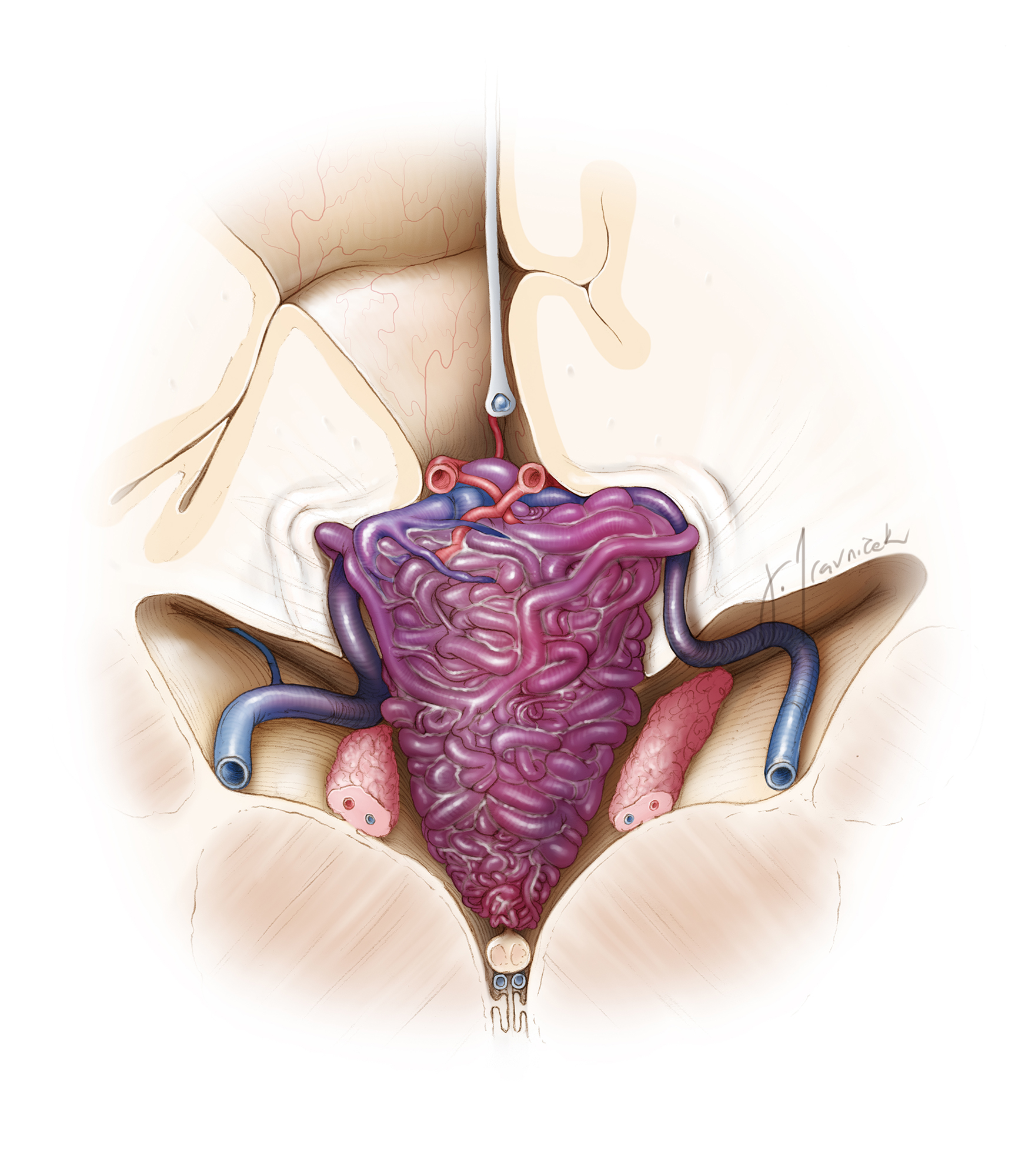
The lateral ventricles are divided into five segments: the frontal horn, body, atrium, temporal horn, and occipital horn. The body, atrium, and temporal horn house the choroid plexus, which is contiguous with the roof of the third ventricle, extending posteriorly into the suprapineal region.
An intimate awareness of the eloquent neural structures within and adjacent to the periventricular system is paramount during intraventricular and periventricular surgery. For lateral ventricular surgery, these major structures of interest include the corpus callosum, forniceal bodies, and caudate nucleus.
The corpus callosum is divided into four segments: the rostrum, genu, body, and splenium. The corpus callosum is the largest single structure establishing the walls of the ventricular system. It is located along the superomedial borders of the lateral ventricles. The caudate nucleus is divided into three segments: the head, body, and tail.
The caudate nucleus borders the lateral wall of the lateral ventricles along its head and body. and its tail borders the roof of the temporal horn. The fornix is divided into the fimbria and the body. The fornix, as a component of the limbic system and Papez circuit, contains the hippocampomammillary tracts arising from the hippocampus en route to the mammillary bodies via its anterior pillars.
The choroidal fissure is a C-shaped anatomic cleft between the fornix and thalamus, where the choroid plexus is anchored during its course through the lateral ventricular body, atria, and temporal horn. The choroidal fissure within the body of the lateral ventricle continues into the third ventricular roof and the velum interpositum via the foramen of Monro.
The third ventricle is a midline structure housed between the two thalami and hypothalami. It communicates rostrally with the lateral ventricles via the foramina of Monro, and caudally with the fourth ventricle through the aqueduct of Sylvius. Its roof contains the forniceal body, tela choroidea, the medial posterior choroidal arteries, and the internal cerebral veins (ICVs). Within the third ventricle there are two layers of tela choroidea, separated by the velum interpositum, an invagination of the pia matter.
The main arterial feeders to the supratentorial ventricular system are the branches of the anterior and posterior choroidal arteries. These branches play an important role in supplying the periventricular AVMs and are therefore discussed in detail below.
The anterior choroidal artery arises as the last main branch of the supraclinoid internal carotid artery (ICA) between the origin of the posterior communicating artery and the internal carotid artery termination. It is divided into its cisternal and intraventricular segments.
- Cisternal segment: From its origin at the ICA, the anterior choroidal artery (AChA) courses posteromedially, inferomedial to the optic tract, and into the crural cistern to travel between the cerebral peduncle and uncus. Here it turns rostrally to enter the choroidal fissure. This segment gives rise to highly eloquent arterial supply, including the optic tract, middle third of the cerebral peduncle, posterior limb of the internal capsule, lateral geniculate body, globus pallidus, and optic radiations.
- Intraventricular or plexal segment: This segment begins where the artery enters the choroidal fissure and the temporal horn (the inferior choroidal point) to supply the adjacent choroid plexus. It courses along the medial border of the choroid plexus, anastomosing with the branches of the lateral posterior choroidal artery (discussed below). This segment rarely perfuses eloquent structures compared with its cisternal segment.
Figure 1: The microsurgical anatomy of the anterior choroidal artery (AChA) is shown. The images of the top two rows demonstrate the origin of the AChA via a left-sided subtemporal approach. Note the location of the medial posterior choroidal artery (MPChA). The bottom images disclose the inferior choroidal point after removal of the lateral temporal neocortex. The inferior view of the relevant anatomy of the plexal segment and the corresponding perforating vessels (green arrows) are shown (images courtesy of AL Rhoton, Jr).
The posterior choroidal artery refers to its medial and lateral components:
- Medial posterior choroidal artery (MPChA): This artery commonly arises from the crural section of the postcommunicating segment (P2) of the posterior cerebral artery (PCA), or alternatively from its quadrigeminal segment (P3). It courses along the circumferential extent of the midbrain and travels anteriorly adjacent to the superior colliculus. It then continues rostrally to travel along the roof of the third ventricle, where it resides within the velum interpositum. Next, it enters the lateral ventricle through the foramen of Monro and courses over the floor. The artery gives off vital branches to several eloquent structures throughout its long course. This artery participates in most of the AVMs involving the choroid plexus.
- Lateral posterior choroidal artery (LPChA): Commonly arises from the ambient section of the postcommunicating segment (P2) of the PCA. It then courses through the ambient cistern until it reaches the posterior temporal horn/atrium where it penetrates through the choroidal fissure. Several anastomoses exist between this artery and the medial posterior choroidal artery, as well the anterior choroidal artery.
Click here to view the interactive module and related content for this image.
Figure 2: Please refer to Figure 1 for more anatomical details relevant to the MPChA and LPChA. The top sagittal photo in the current figure illustrates the anatomic relationship of the MPChA to the third ventricle. The middle image demonstrates the more proximal anatomy of the LPChA through the left temporal horn, whereas the bottom photo shows the MPChA anatomy relative to the third ventricle from an inferior point of view (images courtesy of AL Rhoton, Jr).
Figure 3: This illustration further demonstrates the microsurgical anatomy of the MPChA and LPChA. Anastamoses between these arteries on the ventricular wall are frequently seen.
The ventricular venous system can provide important landmarks during the operation. The major veins of interest are as follows:
- Septal veins: Anterior and posterior septal veins drain the medial frontal horn of the lateral ventricle and anastomose with the ICVs.
- Caudate veins: Anterior and posterior caudate veins drain the lateral frontal horn and anastomose with the thalamostriate veins.
- Thalamostriate vein: This vein courses between the caudate nucleus and thalamus, enters the velum interpositum at the posterior margin of the foramen of Monro (angiographic venous angle), and anastomoses with the superior choroidal vein to form the ICVs.
- Internal cerebral veins (ICVs): These paired veins travel within the velum interpositum, originate at the foramen of Monro, and anastomose with the basal veins of Rosenthal and precentral cerebellar veins to form the vein of Galen.
- Choroidal veins: The superior choroidal veins course in the choroid plexus of the lateral ventricle and anastomose with the thalamostriate veins to form the ICVs. The inferior choroidal vein navigates in the temporal horn, anastomoses with the amygdaloid vein and subsequently drains into the basal vein of Rosenthal.
- Atrial veins: Medial and lateral atrial veins drain the medial and lateral walls of the atrium, respectively. The medial atrial vein drains into the ICVs. The lateral vein drains into the basal vein of Rosenthal.
Click here to view the interactive module and related content for this image.
Figure 4: Some of the above mentioned veins in the text are shown in the above photos. The forniceal bodies have been reflected posteriorly in the images of the top and middle rows. A more highly magnified view of the foramen of Monro is demonstrated in the bottom row of images. The columns of fornix form the anterior and superior walls of the foramen (images courtesy of AL Rhoton, Jr).
VENTRICULAR/PERIVENTRICULAR AVM RESECTION
A number of categorization schemes are proposed for ventricular AVMs. I will discuss the callosal, ventricular body, atrial, and temporal horn AVMs as described by Lawton.
Callosal AVMs
Callosal AVMs are the most common subtype of ventricular/periventricular AVMs. These lesions typically occupy the corpus callosum alone, but can extend into the cingulate gyrus superiorly or extrude into the lateral ventricle inferiorly.
Patient positioning and the initial stages of the operation are summarized in the Interhemispheric Craniotomy chapter. I place the patient in the supine or lateral position (right or nondominant side “down”) while the head is turned 90 degrees to align the falx cerebri parallel to the floor. The craniotomy unroofs the superior sagittal sinus. Bone removal extends two-thirds in front and one-third behind the coronal suture to avoid the central lobule for anterior callosal AVMs. Interhemispheric dissection expands the operative corridor toward the midline lesions. Fortunately, parasagittal draining veins do not limit the operative corridor because the draining veins are primarily inferior or posterior to the nidus.
Figure 5: Patient positioning for the interhemispheric approach is illustrated. Note the bulky gel under the contralateral shoulder, relieving the need for excessive neck torsion.
Once the arachnoid adhesions of the interhemispheric fissure and the intercingulate gyri are generously released, gravity retraction of the dependent hemisphere facilitates optimal exposure of the callosal AVM. This retractorless approach along the falx exposes the corpus callosum where the anterior and posterior feeding arteries, including the callosomarginal artery, are identified.
At this point, the surgeon should be mindful of the important cerebrovascular components associated with the callosal AVMs. The anterior-to-posterior location of the AVM along the callosum determines the robustness of the contributions of the anterior cerebral artery (ACA) and posterior cerebral artery (PCA) to the AVM.
For anterior callosal lesions, the primary arterial blood supply arises from the branches of the ACA, including the pericallosal arteries. Venous drainage includes the deep ventricular venous system, including the septal, caudate, and thalamostriate veins, ICVs, and the vein of Galen in splenium lesions.
Next, the anterior and posterior borders of the AVM are inspected for large cortical and callosal feeding arteries. These arteries are meticulously dissected to their point of entry into the nidus and then they are disconnected. Both pericallosal arteries are identified and their en passage branches are preserved.
Figure 6: The surgeon’s interhemispheric view of the malformation is demonstrated. Note the skeletonization of the pericallosal arteries and their feeding vessels to the AVM.
Figure 7: The classic angioarchitecture of a callosal AVM is depicted. Note the draining veins travelling toward the ventricle around the periphery of the AVM. These veins should not be inadvertently injured or coagulated during circumferential dissection.The initial visible surface of the AVM through the operative corridor is only a small part of the AVM’s surface. If the malformation extends to the level of the choroid plexus, the plexal feeders are involved.
I continue to skeletonize the arterial feeders hidden within the sulci. The corkscrew morphology of the large feeding arteries and arterioles provides a clue regarding their identity compared with nonfeeding vessels. The en passage arteries have normal morphology and are small. If the dominant feeders from the ACA and PCA are not disconnected early, the later steps of dissection will be arduous and associated with a significant amount of blood loss.
The dissection continues through the corpus callosum around the nidus as the feeders are identified and coagulated in a sequential and circumferential manner. Intraoperative navigation or image guidance based on computed tomography angiography guides the planes of disconnection and provides another tool for avoiding inadvertent entry into the nidus.
If the AVM extends into the cingulate gyrus, mobilization of the nidus in this territory is necessary. As I continue circumferential dissection of white matter feeders, I enter the ventricle and find ependymal feeders that are expeditiously coagulated and divided to prevent intraventricular hemorrhage. Undue and blind traction on the AVM leads to avulsion of the ependymal and plexal contributory vessels and is therefore not advised.
The deep draining veins are not visualized until the intraventricular margins of the AVM are reached. Eloquence is usually not a limiting consideration in resection of these lesions, but excessive splenial dissection can lead to disconnection syndrome.
The above procedure may be modified to include a posterior interhemispheric craniotomy to expose splenial AVMs.
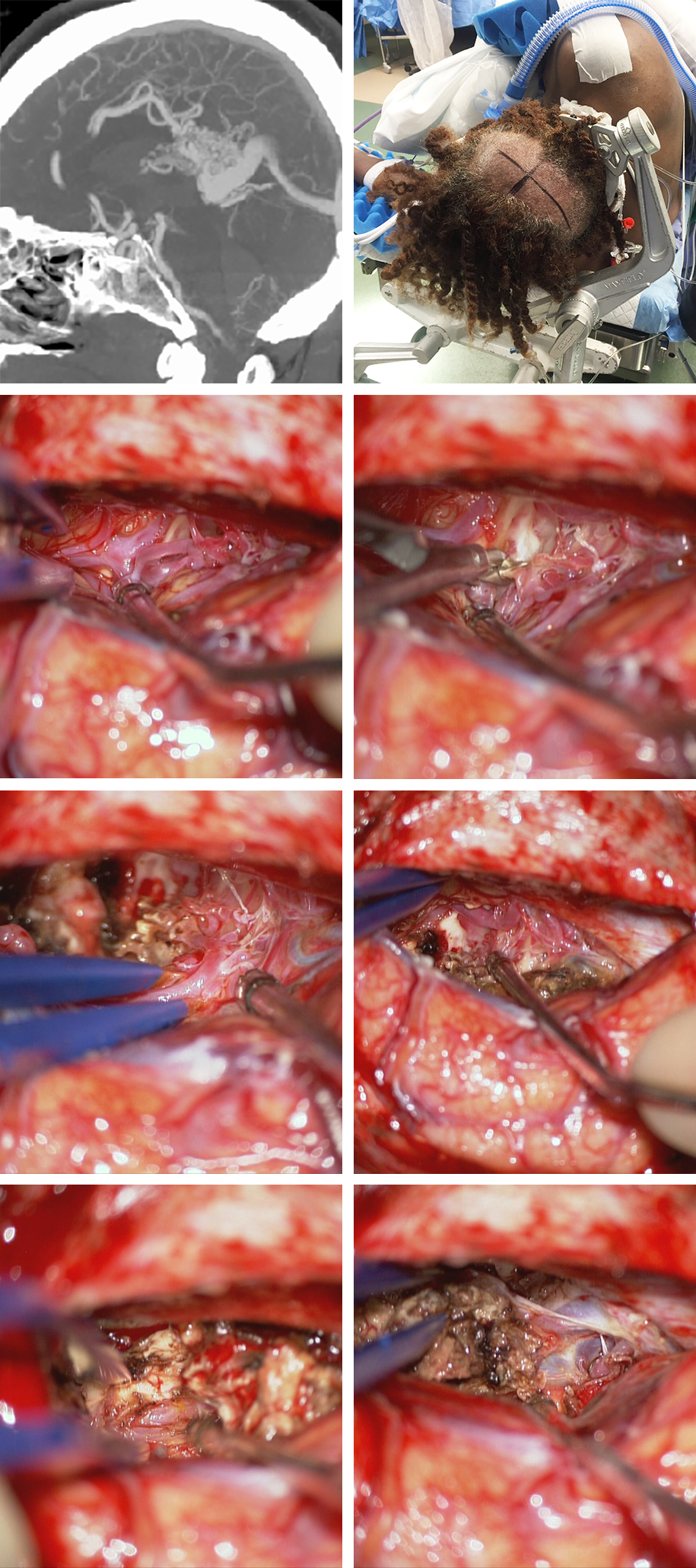
Figure 8: A large hemorrhagic splenial AVM is shown. The lateral CT angiogram demonstrates the ACA feeding arteries and draining veins joining the vein of Galen. The patient underwent a posterior interhemispheric approach via the nondominant side (upper row). The large feeding pericallosal branch was found early and sacrificed early (second row). Cortical cingulate and contralateral pericallosal feeders were also excluded (third row). Ultimately, the PCA feeders were transected and the main draining vein joining the vein of Galen was disconnected.
Ventricular Body AVMs
Ventricular body AVMs are a rare subtype of ventricular AVMs, which are located within the midline along the body of the lateral ventricle at the interface of the adjoining parenchymal structures. This category includes AVMs of the septum, forniceal body, velum interpositum, and ventricular body or third ventricular choroid plexus.
Figure 9: A surgeon’s view of a typical ventricular body AVM is illustrated. Note the numerous feeding vessels from the choroid plexus and the midline septum. The malformation often extends into the foramen of Monro.
Figure 10: This sketch emphasizes the plexal feeders and the direction of the draining veins toward the ICVs.
Similar to callosal AVMs, a right-sided (nondominant) unilateral craniotomy is performed to approach the lesion via an interhemispheric transcallosal approach. For more details describing this route, please refer to the Interhemispheric Transcallosal Approach chapter.
Figure 11: For lesions that extend lateral to the midline, I favor a contralateral transcallosal route to minimize ipsilateral hemispheric retraction and augment the reach around the lateral aspect of the lesion. Malformations of the caudate are approached via the contralateral transcallosal pathway.
Lesions around the third ventricle are safely reached via the transcallosal expanded transforaminal transvenous approach. Once the velum interpositum is exposed, the ICVs and feeding medial posterior choroidal arteries can be identified. The thalamostriate vein can lead the operator to find and protect the ICVs within the velum interpositum.
The ICVs and the medial posterior choroidal arteries are the major venous drainage and arterial feeding sources for ventricular body AVMs, respectively. Lesions located along the septum may also recruit arterial supply from the pericallosal arteries. Progressive circumferential dissection exposes the medial posterior choroidal artery at the posterior margin of the AVM and proximal to the velum interpositum. The anterior communicating artery complex and thalamoperforating arteries may also contribute to the AVM nidus in the form of daunting white matter feeders.
Once the identity of the vessels is verified and their terminal feeding status confirmed, they are occluded. Upon circumdissection of the nidus and identification of the major draining vein joining the ICV, the vein is sacrificed and the nidus removed.
The fornix is closely related to these lesions, presenting a challenge in their safe resection. Overzealous manipulation of the fornix can result in permanent cognitive and debilitating memory impairments.
Ventricular Atrium AVMs
Atrial or trigonal AVMs are the second most common ventricular/periventricular AVM subtype. As the name suggests, they are located within the atrium of the lateral ventricle and most commonly at the medial atrial wall.
A number of surgical approaches have been described for lesions within the medial atrial wall and the trigone. These approaches include anterior-inferior temporal resection, a posterior-inferior temporal resection, parahippocampal resection, paramedian posterior parietal resection, a parasagittal resection, and cingulate resection. The contralateral posterior interhemispheric transfalcine transprecuneus approach (PITTA) to the atrium offers numerous advantages, including a wider operative corridor, minimal ipsilateral brain manipulation, and better intraoperative navigation due to decreased perilesional parenchymal retraction.
Other approaches include anterior trans-sylvian, posterior transcortical/transcallosal, and lateral transtemporal or subtemporal routes. The anterior trans-sylvian approach provides a very narrow corridor to handle highly vascular lesions, and it places motor fibers and optic radiations at risk. The ipsilateral posterior interhemispheric transcortical/transcallosal approach demands significant brain retraction to reach the lateral pole of the lesion. The transtemporal approaches place the optic radiations that line the lateral wall of the trigone at risk. For these reasons, the PITTA is my preferred route to the periatrial lesions.
Another popular surgical approach to the atrium involves the transcortical route via the superior parietal lobule. The patient is placed in a lateral position and the patient’s head turned so that the sagittal suture is parallel to the floor with the patient’s neck flexed laterally to elevate the parietal eminence. Intraoperative navigation guides the craniotomy and the transcortical trajectory.
The primary arterial feeding network of interest for these lesions includes the lateral posterior choroidal artery inferiorly, as well as the anterior choroidal and medial posterior choroidal arteries anteriorly via their corresponding choroid plexuses. The atrial veins that anastomose with the basal vein of Rosenthal and ICV are the primary targets for these lesions.
Figure 12: The vascular anatomy of a classic atrial AVM is shown. Note the feeding vessels from the distal anterior and posterior choroidal arteries. These lesions are intimately related to the choroid plexus that is hypervascular and can lead to excessive bleeding during the AVM’s manipulation. The atrial wall is minimally affected by the mass.
Figure 13: I prefer the contralateral posterior interhemispheric transfalcine transprecuneus approach (PITTA) for reaching the periatrial lesions. In this sketch, the operative view through the more popular transcortical route via the left superior parietal lobule is shown with the sagittal suture parallel to the floor. Note the body of the lateral ventricle is on the inferior aspect of this sketch. The lateral posterior and anterior choroidal arteries provide the dominant feeding arteries to the malformation.
Upon entry into the atrium by means of the PITTA or the transparietal route, the inferior edge of the nidus is targeted and the lateral posterior choroidal arteries are identified, coagulated, and divided. The AVM is mobilized laterally to identify the deep perforators and veins. These are eloquent lesions, given their close proximity to the thalamus medially, crus of the fornix laterally, and internal capsule posteriorly. The optic radiations line the lateral wall of the atrium and aggressive coagulation of the unaffected choroid plexus may lead to ependymal ischemia and visual field deficits.
The interhemispheric transfalcine approach, PITTA, facilitates adequate exposure of the AVM and early proximal control over the choroidal feeders with minimal normal brain transgression. In addition, the PITTA affords numerous working angles to manipulate the vascular lesion without a need for fixed retractors.
The interhemispheric corridor, unlike the transcortical routes, provides landmarks for operative orientation to nearby structures. Transtemporal or other transcortical approaches potentially offer more risk, partly because the surgeon has limited control over the feeding vessels early in the operation.
Figure 14: The use of the PITTA for resection of an AVM on the medial atrial wall and extending into the atrium is shown (top images). The patient was placed in the lateral position with the head turned 45 degrees toward the floor (second row). The right-sided posterior interhemispheric transfalcine approach is shown. Note the straight sinus within the leaflets of the falcotentorial dura just lateral to the tip of the suction device (third row). The primary draining vein is immediately identified over the corpus callosum and a cortical incision in the precuneus is made (fourth row). The AVM is skeletonized and the atrium is entered at the conclusion of nidal circumdissection (bottom row).
Atrial/Splenial AVM Resected via theTransfalcine Route
Diffuse Peri-Atrial AVM Resected via theSuperior Parietal Lobule Approach
Temporal Horn AVMs
Temporal horn AVMs are partially or mostly intraventricular and best managed via a limited (3.5cm from the temporal tip) anterior temporal (middle and inferior gyri) neocortical resection. The anatomic relationship of the Meyer’s loop to the temporal horn requires special mention. The optic radiations travel over the roof of the temporal horn and are especially vulnerable if the extent of the lobectomy is extended more than 3.5 cm from the temporal tip. For more information, please refer to the Anteromedial Temporal Lobectomy chapter.
A tangential anterior-to-posterior operative trajectory within the temporal horn reaches the posterior pole of the malformation. The venous drainage and dominant arterial supply are not identified until the deep and medial poles of the lesion are exposed via the choroidal fissure.
Figure 15: Temporal horn AVMs are technically challenging to tackle because the nidus is covering the feeding vessels emerging from the anterior choroidal artery within the choroidal fissure. I mobilize the nidus as safely as possible without its intrusion to secure some control over the choroidal feeders. The involvement of the anterior choroidal artery often renders preoperative embolization unsafe.
The intraventricular or plexal segment of the anterior choroidal artery provides the dominant feeding pedicle to these lesions. The lateral posterior choroidal arteries provide an alternative contribution. The major venous drainage system consists of the inferior choroidal vein, anastomosing into the basal vein of Rosenthal via the inferior ventricular vein.
After the temporal horn is unveiled and the poles of the malformation identified, I carefully mobilize the malformation and dissect the choroidal fissure, localizing the anterior choroidal artery proximal and the lateral posterior choroidal artery distal to the nidus. During my proximal-to-distal transchoroidal dissection, feeding vessels are first incontestably identified and then coagulated sequentially until the deep venous draining vessels are reached.
During these maneuvers, the nidus is mobilized superiorly so that the medial feeders from the proximal PCA are also identified. Upon identification of the AVM’s venous input to the basal vein of Rosenthal, the vein can be occluded and the nidus subsequently removed. Temporal horn AVMs typically do not infiltrate the hippocampus, and judicious choice of dissection planes furnishes rewarding operative outcomes, despite the close proximity of the mass to the eloquent medial dominant temporal lobe structures.
The distal en passage segment of the anterior choroidal artery should be preserved via meticulous dissection and compulsive identification of the feeders to the malformation. Injury to this en passage segment leads to postoperative hemiplegia.
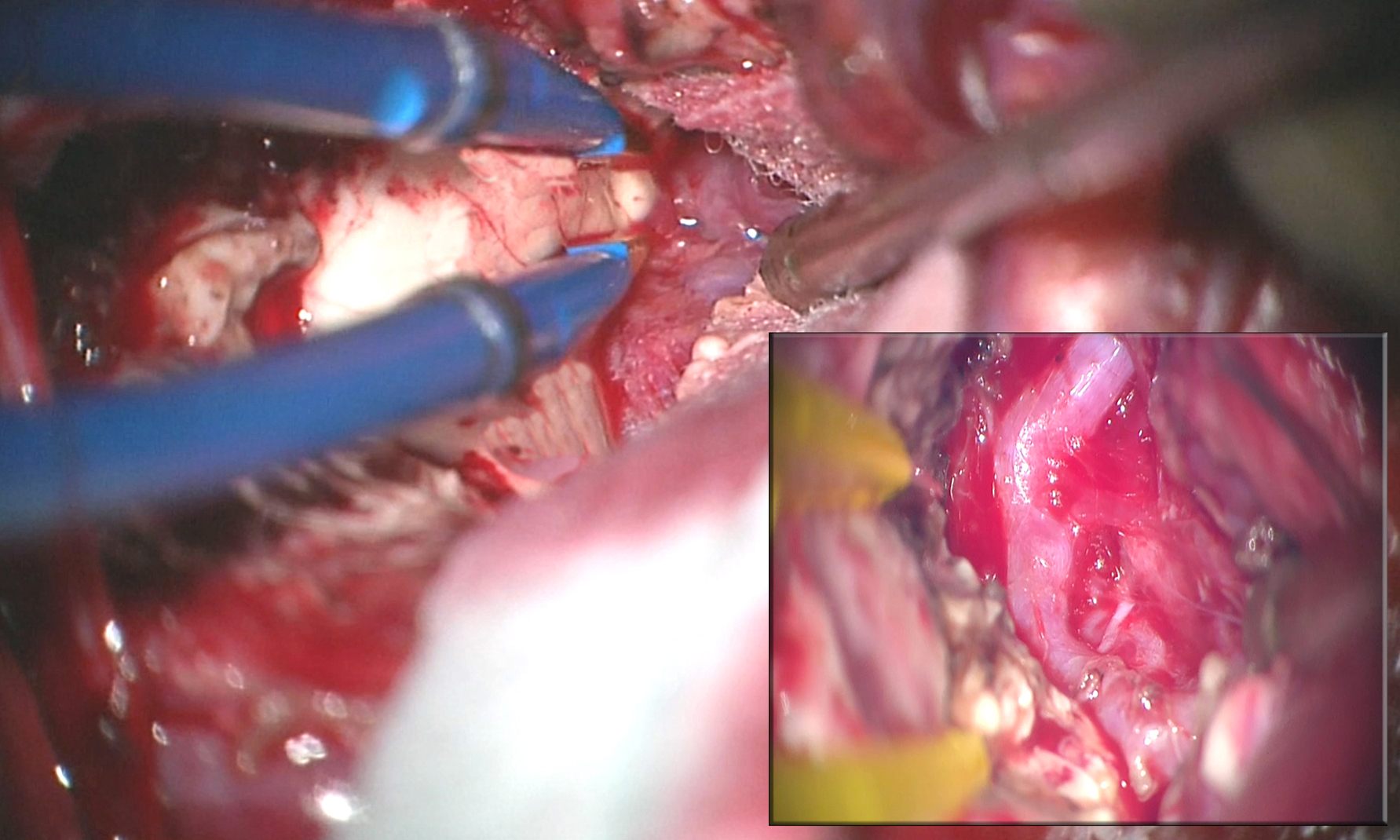
Figure 16: An intraoperative photo demonstrates the exposure of a left-sided temporal horn AVM. The lesion extended through the choroidal fissure and was intimately associated with the choroid plexus. Through the choroidal fissure, the hypertrophied feeding anterior choroidal artery was identified (inset).
Large Medial Temporal/Choroidal AVM
Contributors: Rouzbeh Shams-Amiri, MD, Charles Kulwin, MD, Farhan A. Mirza, MBBS, and Benjamin K. Hendricks, MD
References
Lawton MT. Seven AVMs: Tenets and Techniques for Resection. New York, Stuttgart: Thieme Medical Publishers, 2014.
Rhoton AL. The supratentorial arteries. Neurosurgery. 2002;51[Suppl 1]:53-120.
Spetzler RF. Comprehensive Management of Arteriovenous Malformations of the Brain and Spine. Cambridge: Cambridge University Press, 2015.
Please login to post a comment.