High-Grade Glioma
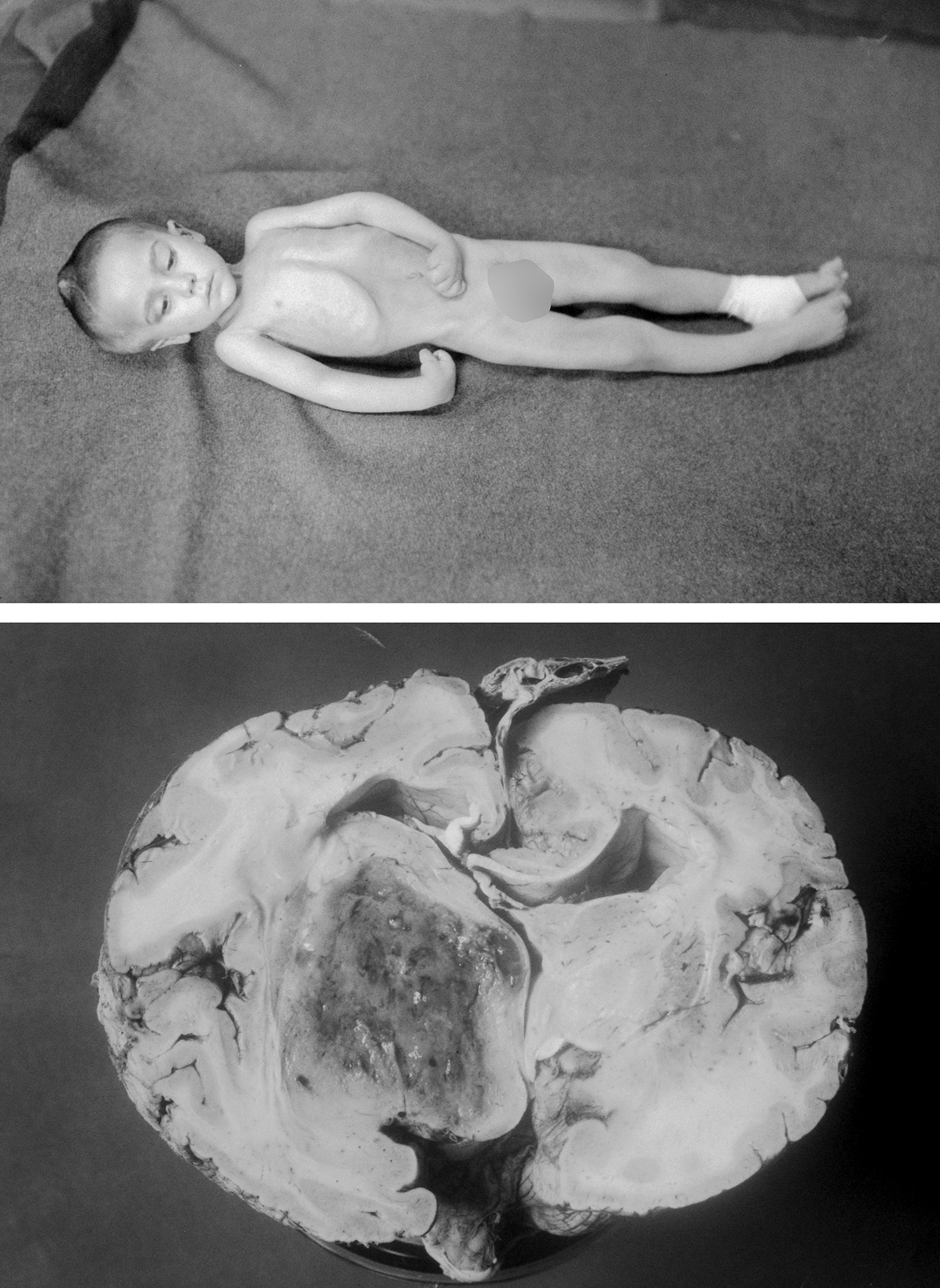
Figure 1: A patient of Harvey Cushing underwent a right parietal craniotomy in 1926 without any remarkable intraoperative findings. Preoperatively, the child was suffering from progressive left-sided hemiparesis. No superficial abnormality was found in surgery. The patient subsequently continued to worsen neurologically: decorticate posturing (upper image-taken by Cushing’s resident-Davidoff). Autopsy brain specimen revealed a thalamic high-grade glioma (Courtesy of Cushing Brain Tumor Registry at Yale University).
High-grade gliomas (HGGs) originate either de novo from glial cells or from low-grade gliomas that dedifferentiate into higher-grade tumors. High-grade gliomas (WHO grade III & IV) include anaplastic astrocytomas, anaplastic oligoastrocytomas, glioblastoma multiforme (GBM), gliosarcomas, and anaplastic oligodendrogliomas.
Primary and secondary GBMs carry distinct molecular genetic profiles. Primary GBMs harbor relatively high frequencies of epidermal growth factor receptor amplification (EGFR), PTEN gene deletion, and CDKN2A (p16) loss, whereas secondary GBMs often contain TP53 and isocitrate dehydrogenase (IDH) mutations.
Despite advances in neuro-oncology and microsurgical techniques, the five-year survival rate for patients with HGG remains less than 10% and the median overall survival is still less than two years. However, the patients who undergo extensive resection and receive adjuvant therapies such as radiation and temozolomide (TMZ) benefit from improved survival rates.
The patient’s age, functional status (Karnofsky performance score-KFS), and tumor histology are the most important parameters in determination of prognosis. Despite numerous investigative efforts, the effect of extent of glioma resection on extending tumor-free progression and patient survival remains unknown. It is also impractical to conduct a relevant randomized study. Most reliable retrospective reports have demonstrated a significant survival advantage with as little as 78% tumor excision, and a stepwise incremental improvement in survival has been demonstrated with additional resection.
It is clear that effective treatments for glioblastoma will be discovered in the laboratory rather than in the operating room. Unfortunately, the biological and molecular heterogeneity of the HGGs (even within the same tumor) is the most limiting aspect of designing effective treatment options.
The goal of surgery remains safe gross-total resection of the enhancing portions of the tumor while preserving function. Any deficit from operative intervention will lead to an undesirable risk/benefit ratio.
These tumors are typically found supratentorially in the frontal, temporal, or occipital lobes. They are often expansive and extend/taper into the deeper structures. The goals of surgery should be thoughtfully planned before surgery, and this is often the most involved part of the surgical process.
Intraoperative neuronavigation and fluorescence techniques, including 5-ALA and fluorescein, have improved the extent of resection. Nonetheless, no significant increase in the overall survival through the use of these technologies has been demonstrated.
I do use navigation and fluorescence routinely and have been able to offer my patients a more effective radical resection of the enhancing portion of their tumor since the use of fluorescence techniques.
Younger patients in good functional status deserve a safe but aggressive treatment paradigm. Patients who are older than 65 years of age seem to do poorly despite radical resection of their enhancing tumors, so these patients may fare better from a biopsy and adjuvant therapy. Multifocal lesions also deserve a less aggressive surgical strategy. Ultimately, every patient’s treatment should be individualized based on a multidisciplinary discussion of his or her case by a team of neurosurgeons, neuro-oncologists and radiation oncologists (Brain Tumor Board).
Diagnosis
Patients with HGGs often present with a variety of symptoms, such as memory dysfunction due to diffuse invasion beyond the boundaries of the enhancing tumor. These symptoms may or may not be helpful in localizing the mass. The family and patient often recall personality changes and memory loss when discussing the history.
Headaches and vomiting are usually related to increased intracranial pressure; the headache location is nonspecific. Seizures may be a presenting symptom or may occur after diagnosis or during treatment. Neurological deficits occur in congruence with tumor location and related vasogenic edema.
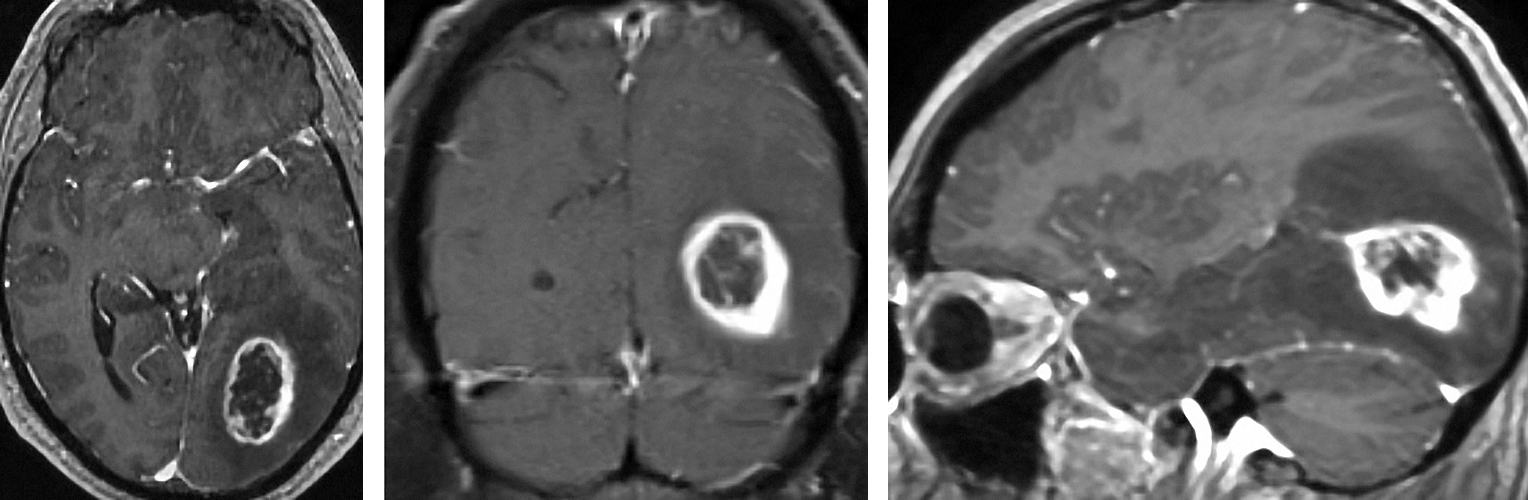
Figure 2: Typical appearance of a ring enhancing lesion consistent with a HGG in the left occipital region. Please note the tail of the tumor leading into the ventricle (right image).
Evaluation
A computed tomography (CT) scan is often the first imaging modality obtained; however, magnetic resonance imaging (MRI) with and without contrast is required to study the pattern of contrast enhancement and extent of the tumor. Gliomatosis cerebri, multiplicity of lesions and leptomeningeal gliomatosis should be excluded before considering extensive resection of any space-occupying lesion.
The size of the tumor is frequently out of proportion to the associated mass effect and edema. Lymphoma, metastatic tumor, radiation necrosis, resolving intracerebral hematoma, recent infarct, multiple sclerosis plaque and brain abscess are frequently included in the differential diagnosis of a ring-enhancing mass. When a nonoperative lesion such as lymphoma or multiple sclerosis is suspected, additional noninvasive diagnostic studies are warranted before a biopsy procedure is contemplated.
An abscess is more likely to be multifocal, frequently leads to markedly more perilesional edema and has a more regular thick enhancing wall. Additionally, the patient harbors constitutional symptoms of infection, such as fever and malaise, and has recent history of contiguous infective sites or hematogenous septic foci. The periventricular location of lymphoma and its more solid uniform enhancement pattern can increase the suspicion about its diagnosis.
SPECT (single photon emission computed tomography) and PET (positron emission tomography) scans are helpful and suggestive in the diagnosis of HGG, given their ability to provide a snapshot of cerebral blood flow, but neither is specific enough to supplant or replace a biopsy. MR spectroscopy and MR perfusion are helpful for supporting the diagnosis of recurrent tumor versus radiation effect.
Indications for Surgery
The practical indications for resection (versus biopsy alone) include tissue diagnosis, improvement in symptomatic mass effect caused by the tumor, and an opportunity to improve the patient’s survival. Resection of a single large superficial lesion within the “silent” regions of the brain (i.e., frontal and the right nondominant temporal lobes) in younger patients is indicated. However, resection of tumors in close proximity or within the eloquent areas remains controversial, especially among patients with good performance status.
Procedures that are associated with a high risk of temporary postoperative neurologic deficits are difficult to justify. Patients with poor functional status are unlikely to benefit from resection and should be considered for a biopsy procedure. Older patients (>70 years of age) must be judiciously screened for an aggressive surgical procedure.
Tumors that are multicentric, cross the corpus callosum or originate in deep structures such as the thalamus are not candidates for resective surgery. Some thalamic lesions can undergo adjuvant treatment even without tissue diagnosis as the risk of a biopsy procedure is not insignificant in these locations.
I do not believe deep insular HGGs are safely resectable despite the application of awake mapping techniques because there is a substantial risk of postoperative decline despite preservation of functional tracts and cortices. Young patients with nondominant, more lateral insular HGGs could be considered candidates for surgery using awake mapping strategies.
Removal of HGGs within other functional tracts and cortices (sensorimotor and speech areas) frequently leads to worsening of neurologic function. This can occur despite preservation of these cortices and tracts. For this reason, I am cautious about weighing the risks and benefits of surgery for patients with tumors infiltrating eloquent regions. Unfortunately, these patients, despite our best efforts, have short survival time to recover from even some of their “temporary” postoperative neurologic deficits.
Figure 3: This 62 year-old patient presented with mild speech difficulty and was diagnosed with a HGG around the angular gyrus on imaging. In my opinion, resection of this mass, despite the use of awake mapping techniques, carries a real risk of permanent speech dysfunction. This tumor was only biopsied and further treated with adjuvant therapies.
Figure 4: Highly vascular HGGs are a supermalignant class of tumors. Their subtotal resection is highly risky. This 26 year-old patient presented with headaches and underwent subtotal resection of her left temporal GBM (top, lateral vertebral angiogram demonstrates exuberant angiogenesis and early arteriovenous shunting). Postoperative CT scans (bottom) demonstrated large intratumoral hemorrhage. Although her young age supported a decision for resection, a biopsy may have been a more reasonable approach if gross total resection was deemed unlikely because of the deep extent of the tumor.
Preoperative Considerations
An independent medical neuro-oncological consultation is highly advised before surgery to ensure an independent and frank discussion about the benefits of any operative intervention. This is also an opportunity for the patient and family to discuss postoperative adjuvant therapy options for the tumors in the list of differential diagnoses. The patient should consider enrollment in a variety of treatment protocols; some which require preoperative preparation (i.e., vaccine trials).
Pre-, intra-, and postoperative steroids are administered to minimize brain edema; preoperative antiepileptic medications are also administered and weaned off ~7 days after surgery if there is no evidence of perioperative seizure activity.
Neuronavigation and fluorescence technologies are adjuvant tools to enhance gross total resection of the enhancing portions of the tumor. I do not use intraoperative MRI because the combination of neuronavigation and fluorescence provides the necessary tools for radical and efficient resection of enhancing tumors.
RESECTION OF HIGH-GRADE GLIOMAS
The craniotomy is planned based on the location of the tumor and neuronavigation data. Frontal and temporal exposures are most needed given the high frequency of HGGs within these lobes. Please refer to the Cranial Approaches volume for more details.
Unfortunately, nearly all patients with HGG suffer from tumor recurrence and some require a repeat resection operation. When planning the initial skin incision, it is therefore prudent to plan for likely future craniotomies within the areas of possible recurrence, including the margins of the resection cavity. Typically, the tumors recur from the anatomical points where the surgeon had the least access during the initial surgery.
Linear incisions and their wide vascularized pedicles offer the most flexibility for extension in repeat operations; they heal most effectively and are at least risk of breakdown after radiotherapy and chemotherapy.
Though small incisions seem appealing, some patients are better served by a generous incision leading to an adequate operative corridor for maximal resection. The associated edema often demands a larger bony opening during craniotomy in order to prevent cortical herniation and infarction along the bone edges that can obscure the surgeon’s view.
INTRADURAL PROCEDURE
Resection of gliomas is not technically challenging. Basic principles and techniques for resection will be reviewed.
Figure 5: The dura may be incised in either a curvilinear or stellate fashion. The opening adequately exposes the surface of the brain so normal tissue is visible on all sides of the lesion. This exposure is verified via navigation. If mapping is contemplated, a more generous craniotomy is indicated to expose the surrounding functional cortices. Hypertrophied veins draining the vascular tumor are often apparent. Gliomas seems to respect gyral borders in certain locations. Thin normal appearing cortex covers the pseudocapsule of the tumor within the white matter. The superficial location of the tumor in the right lower image is marked with an “X” while its subcortical boundaries are marked with the back suture.
Figure 6: The infiltrated gyri are discolored, expanded and appear more vascular than their normal counterparts. Some tumors have highly distinctive pial margins. Subpial coagulation and dissection allow disconnection of the tumor from the surrounding cortices. The surrounding en passage arteries and veins must be carefully protected. I mark the borders of the tumor on the cortex using bipolar cautery before the tumor is debulked. Tumor decompression can render navigation less accurate due to brain shift.
A piece of tumor is sent for histologic examination and confirmation of the preoperative diagnosis.
Figure 7: Given its diffuse nature, especially through the white matter tracts, the tumor edges in HGGs are difficult to fully delineate from the adjacent peritumoral tissue. The necrotic core is clearly distinguishable. However, it is the contrast-enhanced proliferative wall that can resemble peritumoral tissue. This important fact can mislead the surgeon to remove the necrotic core and leave some of the enhancing wall behind.
A pseudo plane may sometimes be present between the contrast-enhancing wall and surrounding tissue. Neuronavigation and fluorescence are effective techniques that can assist with radical removal of the enhancing portions of the tumor. When the resection cavity collapses and its walls fold onto themselves, residual tumor may easily hide within these walls. Fluorescent techniques are especially useful in this situation.
When possible, I prefer to circumferentially disconnect and deliver the tumor en bloc. This maneuver advances technical efficiency, minimizes blood loss, and allows the operator to keep his or her orientation while maintaining the resection planes along the tumor margins. Working simultaneously on the inside and the periphery of the tumor can lead to bleeding and confusion about the tumors’ pseudo margins.
The suction apparatus serves not only as suction but also as a dissector and retractor. It can be used as a vector of dynamic retraction to prevent the wall of the resection cavity from collapsing.
The technique of white matter dissection and disconnection of glial tumors is worth a special emphasis. The bipolar forceps repeatedly grab and coagulate the pseudo capsule. This stepwise maneuver leads to emulsification of the pseudo capsule and its disconnection from the peritumoral edematous tissue. Next, the suction removes this emulsified material and exposes the next layer of the pseudocapsule for further coagulation and disconnection.
The above technique disconnects and coagulates simultaneously. In other words, the bipolar forceps effectively act as tumor scissors by their repeated opening and closing while coagulating (above image, figure 6, inset).
Figure 8: Using bipolar cautery and working evenly around the circumference of the tumor, I extract most of the mass. Further inspection often discloses residual tumor along the folds of the cavity. Small en passage vessels within the sulci at the depth of the cavity are preserved. If the tumor is extending into the ventricle, the resection should expose the intraventricular contents.
Hemostasis is especially important. The most common cause of postoperative hematoma formation is the presence of residual tumor. Therefore, the best method to achieve hemostasis is gross total removal of the tumor. For this reason, I have dampened my enthusiasm for subtotal resection of HGGs that infiltrate highly functional tissues.
I do not line the resection cavity with Surgicel (Ethicon, Cincinnati, OH) as this may disguise bleeding points and give the operator a false sense of security. The use of Surgicel can also lead to delayed postoperative MRI enhancement and create confusion about potential tumor recurrence.
Maximizing GBM Resection: Fluorescence Techniques
Medial Parieto-occipital Glioma: Transfalcine Approach
Closure
I do not insist on a watertight dural closure for supratentorial craniotomies. Tack-up stitches are used to prevent epidural hematoma or fluid collections. The edges of the skin are well approximated.
Postoperative Considerations
A postoperative MRI is obtained within 48 hours of resection to assess for residual tumor. Steroids are weaned slowly as tolerated by the patient. Prophylactic anticonvulsants are administered within the postoperative period, but tapered off within seven days of surgery if there is no evidence of perioperative epileptic activity. I encourage our neuro-oncologists to see the patient before discharge in anticipation of a clinic visit shortly afterwards to discuss the pathology results and plan further treatment strategy.
Patients may undergo radiation therapy two weeks after surgery if appropriate wound healing is evident.
Pearls and Pitfalls
- Careful selection of patients for operative intervention is crucial for desirable outcomes.
- The best method to achieve hemostasis is gross total removal of the tumor. Slow ooze within the cavity indicates residual tumor and a high risk of postoperative hematoma formation.
Contributor: Gina Monaco, MD
References
Tran B, Rosenthal MA. Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci. 2010; 17: 417-421.
Rock JP, Rosenblum ML. The surgical management of high-grade astrocytomas, chapter 34, in Sekhar LN, Fessler RG (eds): Atlas of Neurosurgical Techniques. New York: Thieme Medical Publishers, 2006, 429-439.
Please login to post a comment.