Operative Spinal Cord Anatomy
The spinal cord (SC) is a longitudinal neural structure within the spinal canal, which acts mostly as a conduit for information reaching or leaving the periphery of the body. The SC starts at the foramen magnum and extends to the conus medullaris at approximately the level of the first lumbar vertebra.
The SC is 45 cm in length and is shorter than the spinal canal due to the classical growth mismatch between it and the vertebral column during embryonic development. Thus, in order to maintain the established segmental connections, thoracolumbar roots must elongate to reach their intervertebral foramina.
As a consequence, the upper cervical roots reach their exit almost horizontally to the vertebral level; lower cervical and upper thoracic roots course obliquely one to two segments below; and lumbosacral roots travel almost vertically over several segments in the typical arrangement of the cauda equina.
The SC has a cylindrical shape, which is slightly flattened in the anteroposterior axis. It follows the curvature of the vertebral column and shows two characteristic enlargements, namely the cervical and lumbar ones, where motor neurons related to the upper and lower limbs concentrate. The first is observed at the cervical spinal cord level from C4 to T2, whereas the latter has a close correlation to the first three lumbar vertebrae. Finally, the conus medullaris is usually aligned to the first lumbar vertebra and gives rise to more than 50 rootlets over a length of <3 cm.
The SC is covered by the flexible vertebral column and the meninges. The dura mater is the most external layer, has about 0.8 mm thickness, and contains collagen and elastic fibers. At the spinal level, the dura is arranged in three layers, contrary to the two-layer intracranial dura. The internal layer is in continuity with the inner dural layer of the head; the middle layer is connected to the external dural layer of the head; and the external layer continues as the periosteum of the skull.
In the SC, the outermost or periosteal layer is not attached to the middle layer; this configuration creates an anatomical epidural space that contains veins and fat tissue. The dura covers the entire spinal canal until the level of S1-S2, where the dural sac is formed.
Under and loosely attached to the dura is the arachnoid layer, which contains the subarachnoid space filled with cerebrospinal fluid. It also extends to the dural sac. The arachnoid covers the spinal nerves toward the root sleeves, where it fuses with the dura.
Within the subarachnoid space, several septations have been described, especially in the posterior space, where there is a longitudinal dorsal or dorsolateral septum from the arachnoid to the spinal pial surface dividing the subarachnoid space into left and right halves. It tapers to a fenestrated appearance in the cervical and conus medullaris areas. From the surgical standpoint, such dorsal or posterior septations can facilitate dissection of the extramedullary tumors away from the spinal cord.
The pia mater is the innermost meningeal layer and encases the spinal cord. It provides a barrier between the subarachnoid space and the perivascular spaces. The pia is firmly attached to the dura by 21 pairs of extensions, called the denticulate ligaments. They run alongside the spinal cord to the level of the conus medullaris, where they end between the last thoracic and the first lumbar nerves.
These ligaments are triangular in shape with their bases attached to the surface of the spinal cord, while their apices insert 2 mm dorsal and 3 mm cranial to the intervertebral foramina. The denticulate ligaments are opportunely located between ventral and dorsal roots, are thicker cranially near the cervical spine, and tend to decrease in strength as the SC descends.
Figure 1: Diagram of the cross section of the spinal cord and meninges. The arachnoid layer is attached to the dura. The intermediate layer attaches to the arachnoid and pia mater. A posterior dorsal septum is easily identified. Denticulate ligaments are shown bilaterally.
Figure 2: Photograph of a cadaveric specimen after thoracolumbar laminectomy and midline dural incision. There is a marked change in SC vascularity from the thoracic (left side of photo) to the lumbar SC (right side). The dorsal roots have an oblique trajectory toward the intervertebral foramina. Denticulate ligaments are positioned anterior to the dorsal roots and have a typical bright white appearance.
The understood role of the denticulate ligaments is to promote spinal cord stability within the SC. Conversely to previous belief, the denticulate ligaments limit cranial-caudal movements of the SC, being especially resistant to caudal stress, but have minimal or no significant contribution to limiting anterior-posterior motion.
The pia ends as filum terminale, a fibrous band that connects the conus medullaris with the coccyx. It occurs as a result of dedifferentiation of the caudal-most parts of the SC. Typically, the filum terminale is divided into two distinct segments, namely the intradural part (until reaching the dural sac; called internum) and the extradural part (called externum or ligamentum coccigeale).
Histologically, the filum terminale internum fuses with the fibers of the dural sac and continues as filum terminale externum. From the physiologic point of view, cadaveric strain and elasticity measurements have demonstrated that the filum together with the denticulate ligaments exert a protective role on the conus medullaris from traction.
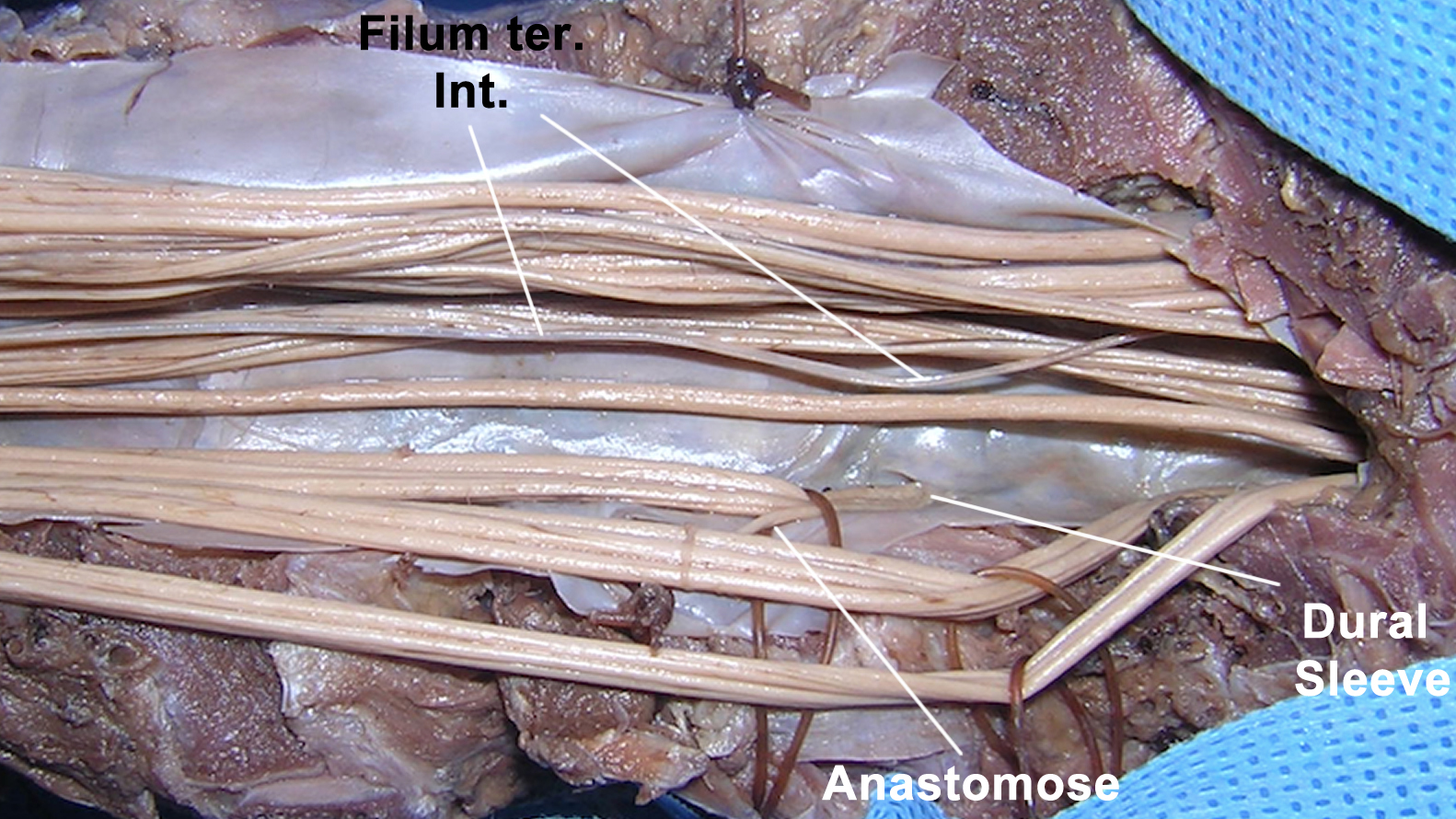
Figure 3: Photograph of a cadaver specimen demonstrates the anatomic relationships of the cauda equina. Typical anastomoses are observed, especially between the dorsal roots on their way to the intervertebral foramina (dural sleeve). The filum terminale internum is also marked. Note the difference in the color of the filum versus the cauda equina.
Figure 4: Intraoperative photograph illustrating the cauda equina. Radicular arteries and the artery of the filum terminale (black arrows) are also noted.
Cross Sectional Anatomy
The SC contains longitudinal columns of nuclei (gray matter), which are enclosed by ascending and descending tracts (white matter). In the axial section, the gray matter demonstrates the widely recognized H-shaped structure. It is divided into anterior (ventral), posterior (dorsal), and, at some levels, the intermediate horns (lateral). Such division is extended to the white matter, revealing the anterior, posterior, and lateral columns or funiculi.
Each segment of the SC contains a ventral and a dorsal root. There are 31-pairs of spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal). From the anterior horn, several motor ventral filaments arise at the anterolateral sulcus and gather to form the ventral root. From the lateral horn, at the level of thoracic and upper lumbar areas, preganglionic sympathetic fibers arise to innervate the autonomic ganglia. Similarly, at the S2-S4 levels, preganglionic parasympathetic fibers arise.
Dorsal sensory afferents enter the posterolateral sulcus on their way to the posterior horn. Deeper fissures are consistently encountered at the ventral (anterior median fissure) and dorsal (posterior median sulcus) surfaces. Moreover, the posterior column is divided by the posterior intermediate sulcus at upper thoracic levels.
In the center of the SC lies the central canal. It consists of the remnants of the neural tube central cavity lined by ependymal cells and filled with cerebrospinal fluid. The anterior and posterior commissures enclose the central canal.
The gray matter horns are somatotopically organized and contain different classes of functional neurons. As a result, motoneurons that innervate axial muscles are medially located in the ventral horn, whereas motoneurons that control distal limb movements are located more laterally. Finally, motoneurons responsible for controlling proximal limb muscles lie in between.
The posterior horn has a layered neuronal organization, which is based on synaptic inputs and outputs. The superficial layers receive exteroceptive sensory information about pain, temperature, and light touch, and generate the contralateral spinothalamic tracts. The deep layers are involved with proprioceptive information and contribute to the ipsilateral spinocerebellar tracts. The posterior cervical horn also includes the spinal nucleus of the trigeminal nerve.
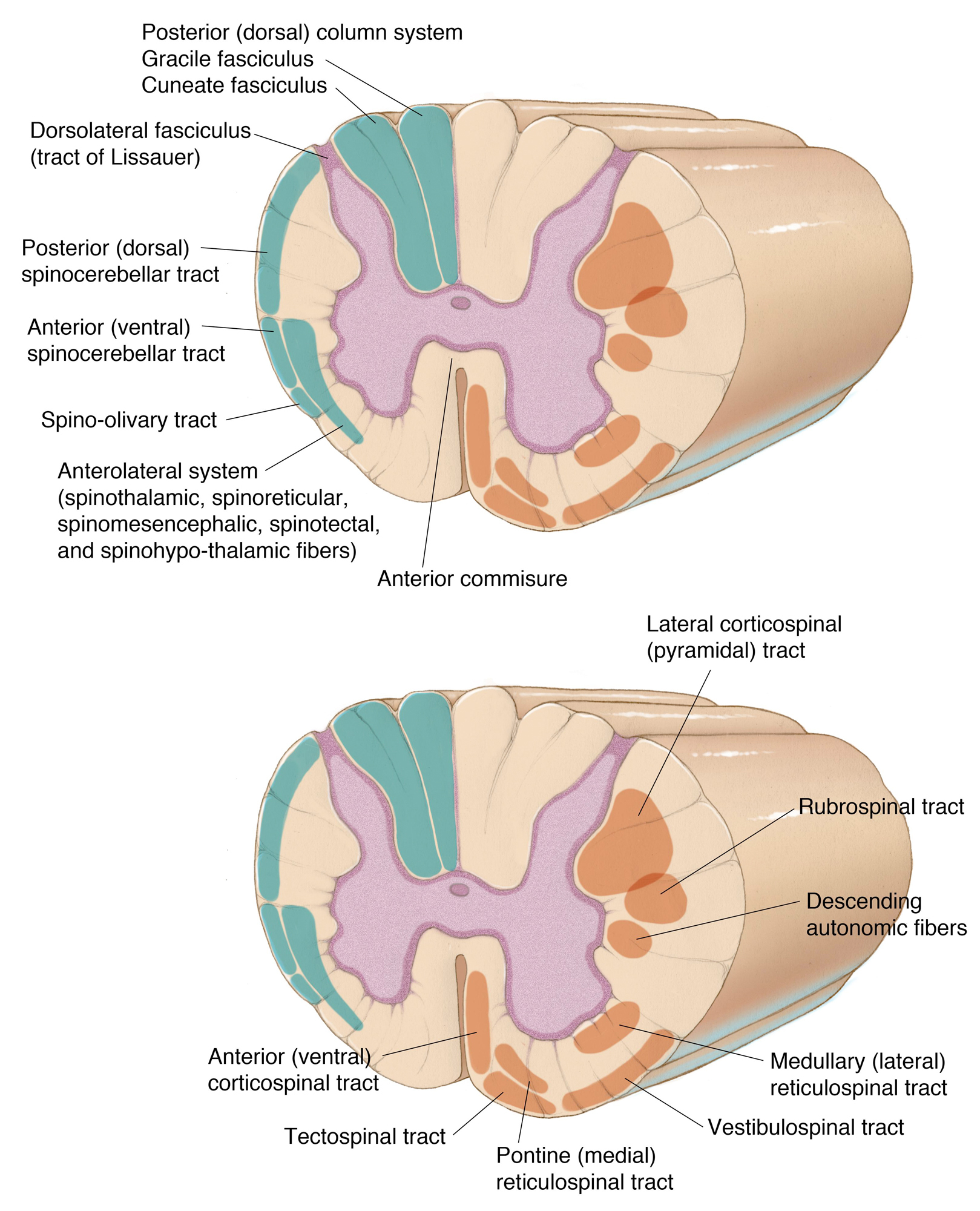
The white matter is organized in tracts associated with major motor or sensory functions. The posterior column enlarges, as the SC ascends, to include more axons carrying fine touch, vibration, and proprioceptive information from the lower limbs medially (fasciculus gracilis) and the upper limbs laterally (fasciculus cuneatus). The lateral column contains two most prominent ascending tracts, namely the lateral spinothalamic and the spinocerebellar ones, and one descending tract, the lateral corticospinal tract. Finally, the anterior spinothalamic and corticospinal tracts are found in the anterior column.
The spinothalamic tracts carry contralateral information about crude touch, pain, and temperature. Two topographical details in these tracts are worth discussing. First, the initial synapse occurs at the posterior horn, where second-order neurons generate axons that course approximately two to three levels above in order to decussate through the anterior commissure. Second, sacral fibers are placed more laterally than cervical ones, thereby justifying the phenomenon of sensory sacral sparing in central SC lesions.
The lateral corticospinal tract is formed at the caudal medulla, where most of the fibers coming from the primary motor cortex decussate. The uncrossed fibers continue as anterior corticospinal tract and decussate only at the level of the synapse with the ventral horn neurons. This tract is involved in the control of proximal limb and axial muscles.
The lateral corticospinal tract shows a similar topographical distribution to the spinothalamic tract, in such a way that sacral fibers are situated laterally. In clinical scenarios, compressive cervical conditions initially affect motor functions of the lower limbs. Several other tracts are described, even though they are less clinically relevant.
Figure 5: Major white matter tracts, ascending (top illustration) and descending (bottom illustration), are noted. In other words, orange tracts are descending pathways and blue tracts are ascending pathways.
Spinal Vascular Anatomy
The arterial network that supplies the SC is much more complex and extensive than that of the brain. The spinal vascular anatomy starts in the segmental extraspinal arteries, which correspond to the pathways of blood from the aorta and provide not only the arterial supply to the cord, but also to the nerve roots, dura, and paraspinal musculature.
Each segmental artery has a ventral and a dorsal branch. The dorsal division gives off a spinal branch, which splits into the retrocorporeal (anterior spinal canal), prelaminar (posterior spinal canal), and radicular arteries. The radicular artery is termed the radiculomeningeal artery when it feeds the nerve roots and dura at every level.
On the other hand, if these arteries take part in the cord vascular network, they are better termed radiculomedullary arteries if they supply the anterior spinal artery (ASA), and radiculopial or posterior radiculomedullary arteries, if they supply the posterior spinal arteries (PSAs) and surface vasocorona of the SC.
Different segmental arteries are found regionally that provide blood supply to the SC and spinal ganglia. In the cervical region, the vertebral, ascending cervical and deep cervical arteries; in the thoracic region, the intercostal arteries; in the lumbar region, the lumbar, iliolumbar and the lowest lumbar arteries; and in the sacral and coccygeal regions, the lateral sacral arteries are involved in the arterial network.
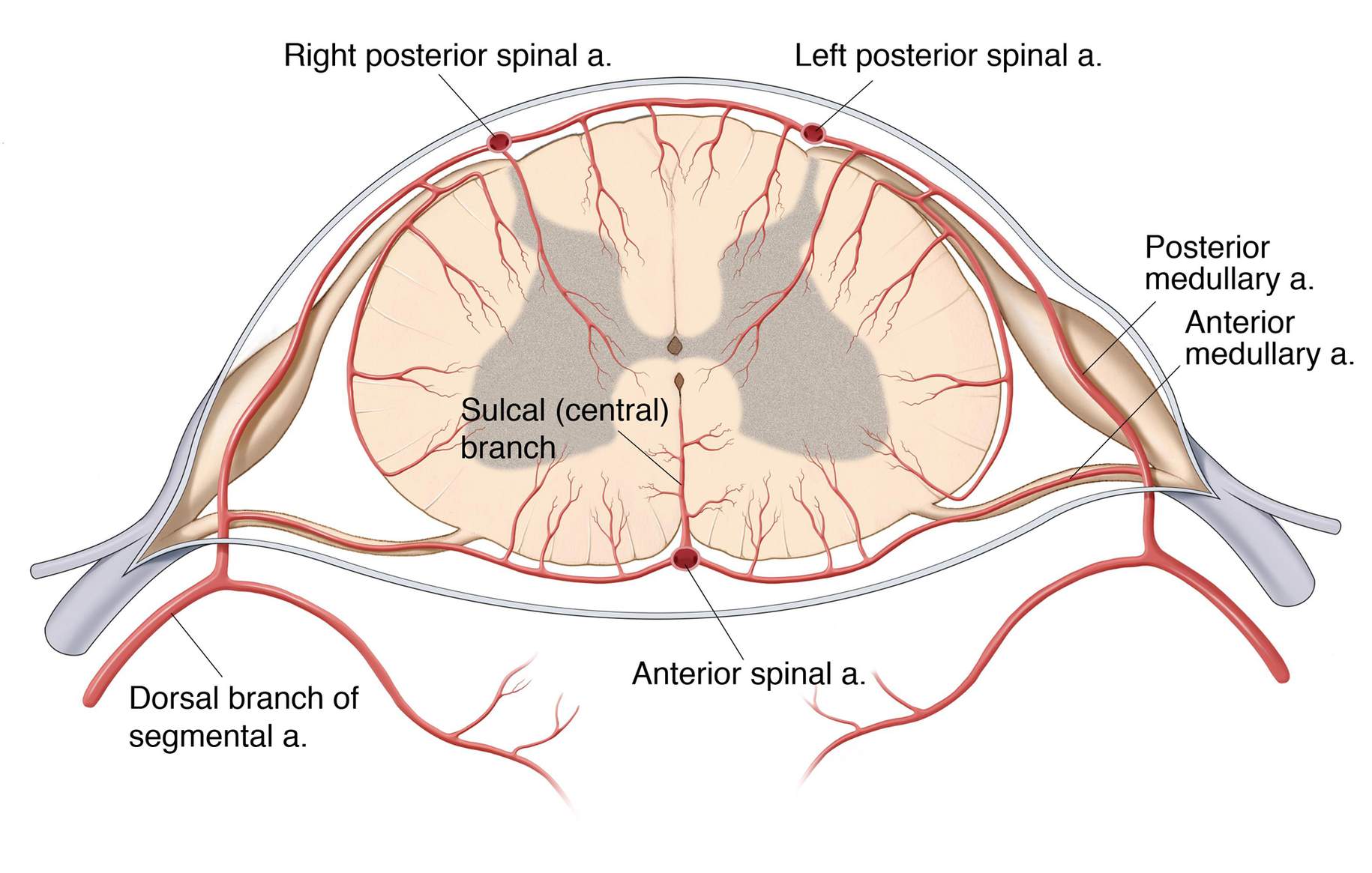
Figure 7: Diagram of the spinal cord vascular anatomy in a cross-sectional view. In this sketch, anterior and posterior radiculomedullary arteries create a complex arterial network with the anterior and posterior spinal arteries, as well as with the vasocorona.
In the cervical region, 8 to 10 unpaired radiculomedullary arteries, including at least 1 medullary artery to the cervical enlargement at the level of C6, connect directly with ASA.
The thoracic region has an otherwise poor vascularity with from 2 to 4 small medullary arteries. The lumbar enlargement at the thoracolumbar region is richly supplied by 1 single vessel, called the artery of Adamkiewicz. It commonly arises between T9 and T12 in 75% of the specimens, even though higher (T5-T8) or lower (L1-L2) levels may also carry this important artery in 15% and 10%, respectively.
The artery of Adamkiewicz is also known as the great radicular artery or even as arteria radiculomedullaris magna and has a left-sided predominance. As the artery pierces the dura, a slight caudal turn may occasionally be seen. The artery then joins the ventral root on its way to the ventral surface of the SC, where the artery anastomoses with the ASA at or just before its typical and characteristic hairpin turn. The PSAs receive approximately 10 to 28 feeders, which can also demonstrate a hairpin configuration in the paramedian locations.
Figure 9: Photograph of a cadaveric specimen (anterior view) showing the typical configuration of the artery of Adamkiewicz. This artery enters the spinal canal on the left side, follows the ventral root, courses over the spinal cord through several segments, and makes a hairpin turn at or just before encountering the anterior spinal artery. (Adapted from Alleyne et al, 1998 with permission.)
For the spinal arterial network, 3 superficial spinal arteries are identified all the way down to the level of the conus medullaris over the anterior median fissure and posterolateral sulci, respectively. They receive significant contribution of several feeders, as mentioned before.
The ASAs originate as medial branches of the vertebral arteries and become a single artery at the midcervical level. The additional 2 superficial vessels (PSAs) originate from the vertebral arteries or even from the posterior inferior cerebellar arteries at the level of the foramen magnum. The anterior two-thirds of the SC is supplied by the ASA, whereas the posterior one-third is fed by the PSAs.
The anterior and posterior systems join at the conus medullaris to form the arterial basket with extensions alongside the filum terminale. The arterial basket consists of at least 1 or 2 anastomotic branches between the ASA and PSAs.
One additional anastomotic system is observed over the SC surface forming a rather distinct pial plexus, which is called the vasocorona. The intramedullary stage of the vascular supply is served by 2 systems, namely the sulcal or central arteries and the radial perforating arteries.
Figure 10: Photograph of a cadaveric specimen illustrating the typical arterial anastomotic network at the conus medullaris. The anterior spinal artery (ASA) forms an arterial basket with the posterior spinal arteries (PSAs) via the anastomic branches (ABCM). Early branching and unilateral configurations may be observed occasionally. A: anterior view; B: right oblique view; C: left oblique view; D: posterior view. (Adapted from Martirosyan et al, 2015, with permission.)
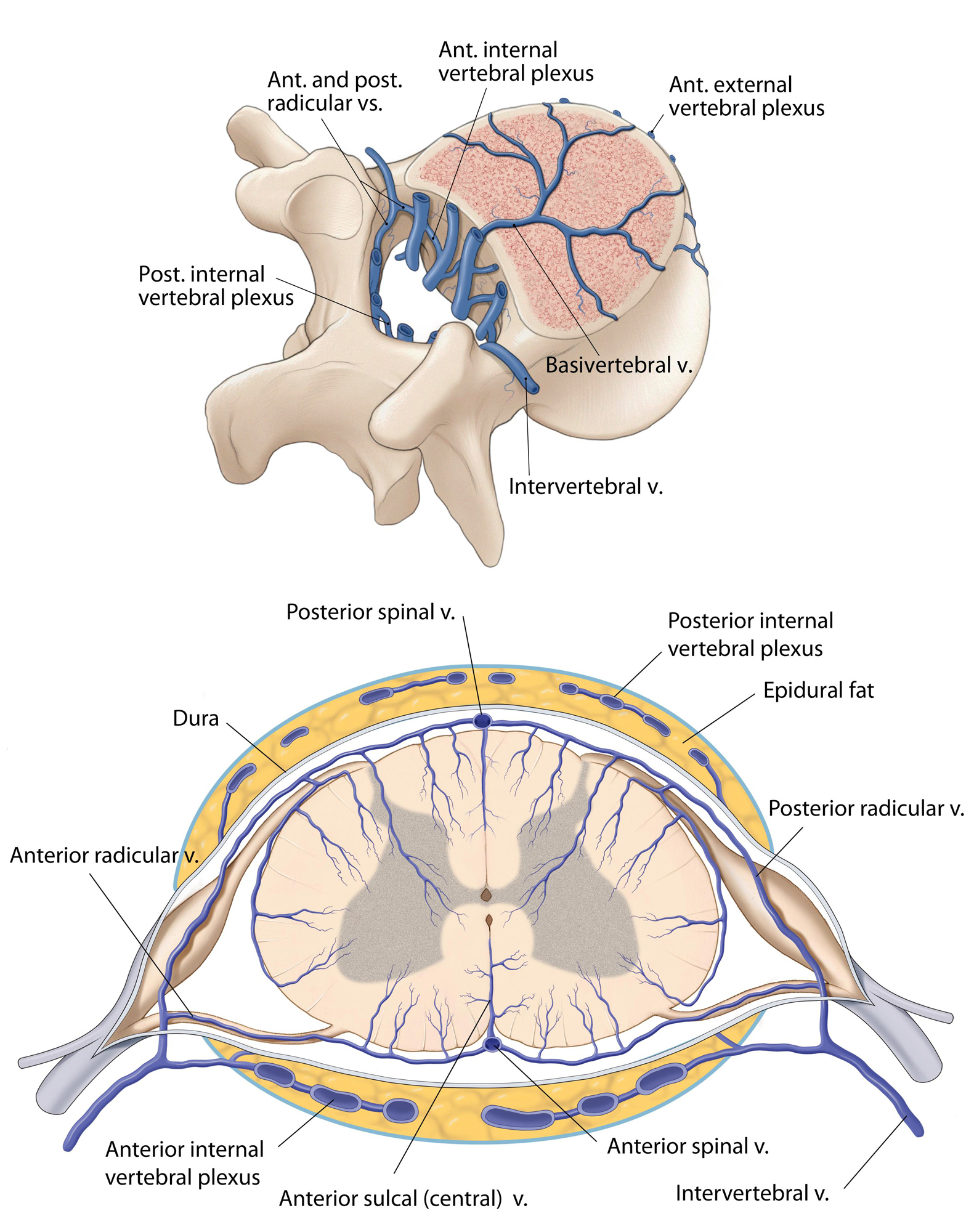
The venous drainage of the SC is more variable than the arterial supply and has been divided into intrinsic and extrinsic systems. The intrinsic system contains centrifugally oriented peripheral or radial veins, which emerge from capillaries at the gray-white matter junction.
Sulcal or central veins drain the anterior horns, anterior commissure, and associated white matter and complete the intrinsic system of venous drainage. The extrinsic system of venous drainage has a direct correlation to the patterns of the arterial system. Two dominant veins are usually observed, the anterior and posterior median veins. The first receives venous drainage from the sulcal veins, whereas the latter receives venous drainage from the peripheral veins.
Next, the anterior and posterior median veins drain into 8 to 14 anterior and 5 to 10 posterior radiculomedullary veins, which in turn empty into the epidural venous plexi through a valveless system. Similarly to that observed in the arterial system, a great anterior radiculomedullary vein owns a crucial role in draining the anterior thoracolumbar spine. It is commonly detected between T11 and L3.
The next stage is the connection to the intervertebral veins and subsequently to the segmental veins (ascending lumbar and azygos systems), before joining the superior vena cava.
Pearls and Pitfalls
- The SC has a complex anatomical organization for receiving, processing, and transferring information.
- Long tracts generally cross the midline, even though decussation occurs at different levels. Depending on the axial and longitudinal extent of the lesion, different motor and sensory syndromes may develop.
- The existence of sulci and fissures over the SC surface provides relatively safe entry routes for reaching intramedullary tumors.
- When approaching the lower thoracic SC levels, the surgeon should investigate the location of the artery of Adamkiewicz.
Contributor: Marcus André Acioly, MD, PhD
References
Alleyne CH Jr, Cawley CM, Shengelaia GG, Barrow DL. Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J Neurosurg. 1998;89:791-795.
Bosmia AN, Hogan E, Loukas M, Tubbs RS, Cohen-Gadol AA. Blood supply to the human spinal cord: part I. Anatomy and hemodynamics. Clin Anat. 2015;28:52-64.
Cho TA. Spinal cord functional anatomy. Continuum (Minneap Minn). 2015;21(1 Spinal Cord Disorders):13-35.
De Vloo P, Monea AG, Sciot R, van Loon J, Van Calenbergh F. The filum terminale: a cadaver study of anatomy, histology, and elastic properties. World Neurosurg. 2016;90:565-573.
Hauck EF, Wittkowski W, Bothe HW. Intradural microanatomy of the nerve roots S1-S5 at their origin from the conus medullaris. J Neurosurg Spine. 2008;9:207-212.
Klekamp J, Samii M. Surgery of Spinal Tumors. Berlin, Germany: Springer, 2007.
Martirosyan NL, Kalani MY, Lemole GM Jr, Spetzler RF, Preul MC, Theodore N. Microsurgical anatomy of the arterial basket of the conus medullaris. J Neurosurg Spine. 2015;22:672-676.
Nicholas DS, Weller RO. The fine anatomy of the human spinal meninges. A light and scanning electron microscopy study. J Neurosurg. 1988;69:276-282.
Rossignol S. Anatomy and phisiology of the spinal cord, in Fehlings MG, Boakye M, Vaccaro AR, Rossignol S, Dittuno A Jr Burns AS (eds): Essentials of Spinal Cord Surgery: Basic Research to Clinical Practice. New York-Stuttgart: Thieme, 2013, pp. 3-17.
Singh P, Gobin P. Spinal vascular anatomy and implications for treatment of arteriovenous malformations in Spetzler RF, Kondziolka DS, Higashida RT, Kalani MYS (eds): Comprehensive Management of Arteriovenous Malformations of the Brain and Spine. Cambridge: Cambridge University Press, 2015, pp. 29-36.
Tubbs RS, Salter G, Grabb PA, Oakes WJ. The denticulate ligament: anatomy and functional significance. J Neurosurg. 2001;94(2 Suppl):271-275.
Please login to post a comment.