Hemangioblastoma
This is a preview. Check to see if you have access to the full video. Check access
Cerebellar Hemangioblastoma: Fluorescence
Hemangioblastomas are benign, World Health Organization (WHO) class I tumors that predominantly (>90%) occur within the posterior fossa. They constitute only 8% of adult posterior fossa tumors and only 1% of all intracranial tumors. These tumors typically originate near the fourth ventricle in the cerebellar hemispheres, vermis, or even the brainstem.
Up to 30% of the patients with hemangioblastomas suffer from Von Hippel Lindau (VHL) syndrome. Although rare, spinal hemangioblastomas can also occur, usually near the cervical or thoracic spinal cord. Most spinal hemangioblastomas are associated with a syrinx and can be easily overlooked in a patient who presents with posterior fossa symptoms.
Patients with a solitary hemangioblastoma lesion have excellent prognosis, and a complete resection of the nodule is considered a cure. However, if the lesions are multicentric, as in patients with VHL syndrome, metastases can occur and the prognosis is poor. This autosomally inherited disease not only can cause malignancies, most commonly in the posterior fossa and kidneys, but can also lead to tumors throughout the body. Penetrance is 70%, so screening of family members with noninvasive tests is warranted.
Posterior fossa hemangioblastomas are typically discovered among patients who are in their 30s or 40s, with nonsporadic lesions becoming symptomatic earlier than spontaneous ones. These tumors are rarely identified in geriatric patients, but because they are so unusual in this patient population, they may be misdiagnosed as metastatic disease. The diagnostic process should include tissue biopsy for pathologic diagnosis if a metastatic work-up is not revealing, unless the patient is at high risk of medical complications from the surgery.
Diagnosis
Patients with tumors in the posterior fossa often present with headaches. Hydrocephalus in these patients is usually caused by compression of the 4th ventricle or mass effect in the region of the foramen magnum. In addition, vomiting is accounted for by hydrocephalus or direct pressure on the area postrema. A variety of cerebellar and brainstem signs and symptoms may also occur based on the exact location and size of the lesion.
More common symptoms may include ataxia, gait dysfunction, vertigo, papilledema, nystagmus, and diplopia. If the patient suffers from VHL syndrome, hemangioblastomas may be found in the spinal cord and may cause symptoms with a similar time of onset as the cerebellar signs. This phenomenon can complicate the clinical picture, and symptomatic spinal lesions may be overlooked, accounting for a lack of neurologic improvement after operative intervention.
Evaluation
Radiographic diagnosis is made via magnetic resonance imaging (MRI). The tumor is usually cystic and contains a mural nodule. The nodule is isointense to the brain intensity, but displays distinct contrast enhancement. The cyst contents closely resemble cerebrospinal fluid (CSF). The enhancement pattern of the cyst capsule is very important because it drastically changes the differential diagnosis and treatment strategy.
The capsule of the hemangioblastoma is composed of glia and will not enhance. This is in contradistinction to a cystic astrocytoma, which displays cyst wall enhancement. The enhancing nodule should undergo resection for hemangioblastomas, whereas the entire enhancing capsule must be resected for an astrocytoma to achieve any chance of cure.
An angiogram of the vertebral circulation may be helpful in diagnosis as the hemangioblastoma nodule is frequently and distinctly more vascular than other tumors. Similarly, an angiogram of a spinal hemangioblastoma can help with characterization and localization if the MRI presents a confusing picture.
Figure 1: The upper images show a typical cystic hemangioblastoma with a prominent nodule. The bottom images illustrate a hemispheric variant with more solid than cystic components.
Figure 2: These images show a purely solid brainstem tumor (left upper image). The corresponding computed tomography (CT) angiogram (right upper image) and anteroposterior and lateral angiograms (lower images) show the extent of tumor vascularity. This tumor can present a complex set of challenges during its resection and should be surgically approached as a high-flow brainstem arteriovenous malformation (AVM) rather than a typical hemangioblastoma in the cerebellar hemisphere.
Patients who are suspected to harbor a hemangioblastoma should undergo spine imaging. In addition to a spine MRI, a comprehensive fundoscopic exam after the pupils are dilated is necessary for VHL patients. To study the pancreas and kidneys, abdominal CT scans with and without contrast are necessary. The physician should rule out hemangioblastoma-induced polycythemia by ordering a complete blood cell count. Of course, a complete workup also includes a comprehensive family history and physical exam.
Indications for the Procedure
Single, symptomatic, and superficial lesions should be resected. Deep-seated lesions in patients with minimal symptoms should be observed. For the surgical management of multiple hemangioblastomas in VHL patients, only the symptomatic or large and easily accessible tumors are removed. Nonsymptomatic or small (~1 cm or smaller) lesions in this patient population should be followed expectantly.
Large and solid posterior fossa hemangioblastomas offer a daunting challenge in their resection because they behave like high-flow AVMs. These high-risk lesions should be approached earlier in their symptomatic course because their natural history is often consistent with rapid growth.
Preoperative Considerations
The precise location of the tumor and its relation to the vital structures of the posterior fossa should be characterized on preoperative imaging. Attention should be paid to the tumor’s relation to the brainstem, fourth ventricle, and the posterior inferior cerebellar artery (PICA), because the PICA usually provides the dominant feeding arteries to these tumors. These feeders should be interrupted early in the procedure, if possible.
The surrounding vital structures mentioned above guide the trajectory of the operative corridor. A cerebellar hemispheric cystic tumor with a mural nodule is the simplest to remove and carries the lowest risk (mortality <2%), whereas a large solid tumor in or near the brainstem is the most difficult and carries up to 50% mortality risk.
The overall surgical management of patients suffering from VHL syndrome can be difficult and complex. As a rule of thumb, removing a malignant renal tumor or pheochromocytoma, which can cause a life-threatening catecholamine surge during surgery, should take priority over removing a benign cerebellar lesion. An exception to this rule is a situation when obstructive hydrocephalus is present, and if so, the compressive posterior fossa tumor should be removed first.
An upper cervical spinal and brainstem tumor accompanying a cerebellar hemangioblastoma can sometimes be removed concurrently through the same incision. Small, deep-seated lesions may be difficult to locate, even while using intraoperative navigation and doppler ultrasonography. If this difficulty is anticipated, more harm than good may come from the operation and observation may be a more practical plan.
In general, preoperative embolization is not a consideration because of the multiple small feeders associated with these tumors. However, in the case of a large, solid tumor, it is highly advisable to attempt embolization because large feeders are not readily accessible intraoperatively and the risk of substantial intraoperative blood loss is significant.
RESECTION OF POSTERIOR FOSSA HEMANGIOBLASTOMAS
Most hemangioblastomas are exposed through a suboccipital, retromastoid, midline supracerebellar, paramedian supracerebellar, or telovelar approach. Please refer to the corresponding chapters in the Cranial Approaches volume for further details.
Because solid hemangioblastomas behave much like AVMs, their exposure must be generous and allow access to the feeding arteries and draining veins. For cystic tumors, the wall of the cyst is not manipulated and therefore may not be completely exposed; however, the nodule must be thoroughly uncovered.
INTRADURAL PROCEDURE
If the dura is found tense after completion of the craniotomy, the cyst may be punctured and drained under navigation or ultrasound guidance using a blunt ventricular needle to relax the brain. This maneuver allows the cerebellum to fall away from the dura and decreases the risk of cerebellar herniation through the dural opening. Obviously, the needle should not disturb the targeted vascular lesion. Once the cyst is located, finding the mural nodule may or may not be difficult.
Figure 3: Exposure of the vascular nodule (X) within the cyst cavity for the images in Figure 1 (upper photos). This lesion was exposed through the telovelar approach.
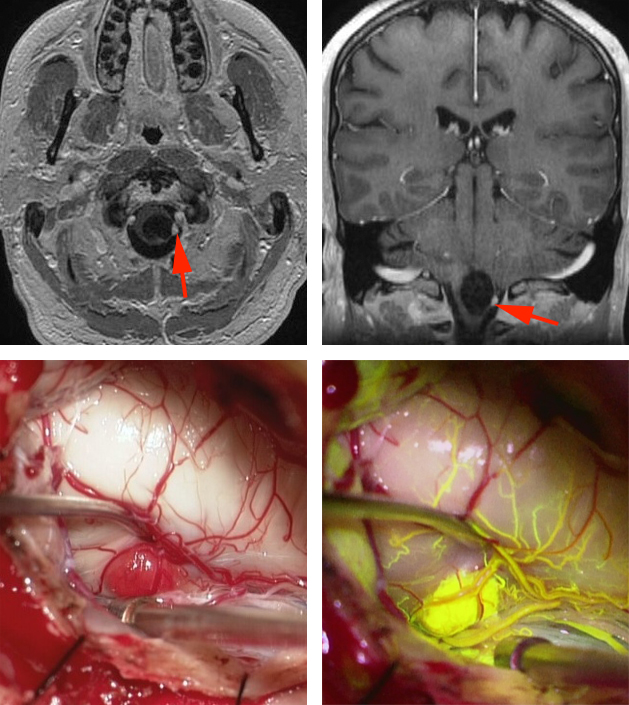
Figure 4: A cystic hemangioblastoma of the medulla oblongata is shown in the upper MR images (red arrows). Intraoperative findings, including fluorescein angiography, demonstrate the angioarchitecture of the feeding arteries and draining veins of the malformation (lower photos).
Brainstem Hemangioblastoma: Fluorescence
The following illustrations highlight the tenets for resection of a right-sided lateral cerebellar cystic hemangioblastoma. These concepts also apply to the same type of cystic tumors in other locations.
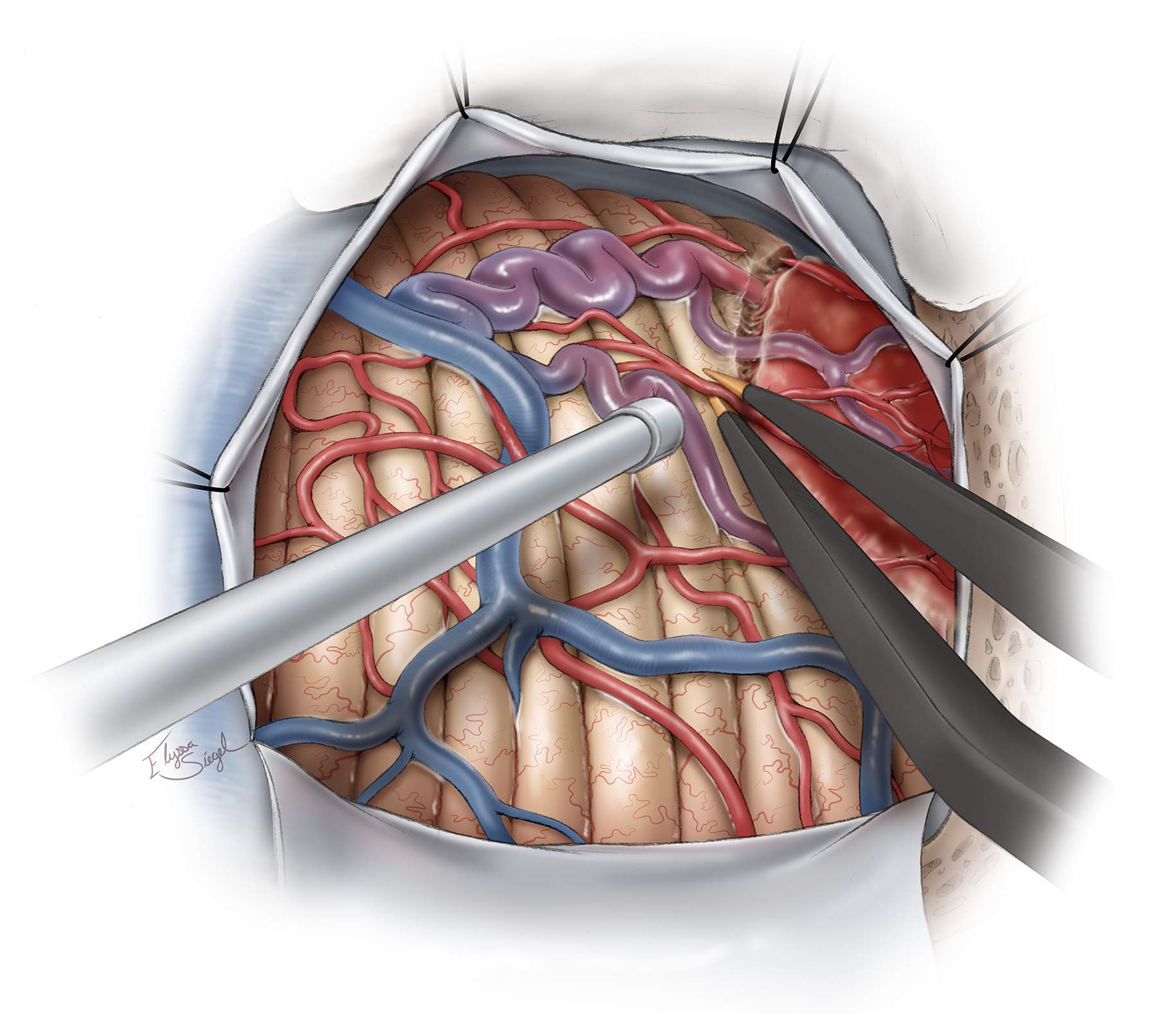
Figure 5: Intradural exposure and identification of the nodule. Nodules may protrude through the cerebellar cortex or reside just under the surface while discoloring the pial surface and recruiting feeding arteries and draining veins. They may be deep and difficult to find if the cyst wall is collapsed. If the nodule is not on the surface, but instead on a deeper aspect of the cyst, it may be flattened or nondistinct in color or shape, making it challenging to localize. The feeding arteries and draining veins of the nodule, reminiscent of an AVM angioarchitecture, are apparent (upper image). The vascular anatomy of the lesion is better demonstrated through fluorescein angiography (right lower image). The nodule is uniformly highlighted.
Figure 6: The boundaries of the nodule are identified using its discolored surface appearance and the presence of the entering feeding arteries and exiting draining veins. Bipolar electrocautery is used to isolate the nodule from the feeding arteries. The operator should not enter the nodule prematurely and should employ the microsurgical principles described for AVM resection. If the nodule is inadvertently entered, a small piece of cotton and tamponade may be used to achieve hemostasis. Alternatively, gentle bipolar coagulation may be applied. The surgeon should continue to circumferentially disconnect all of the arterial feeders and preserve all of the veins that may be arterialized.
If the module is not well vascularized through large feeding vessels, the flow within the nodule is tame and the nodule may be excised efficiently, similar to a tumor. However, prominent feeding vessels demand special attention for their disconnection.
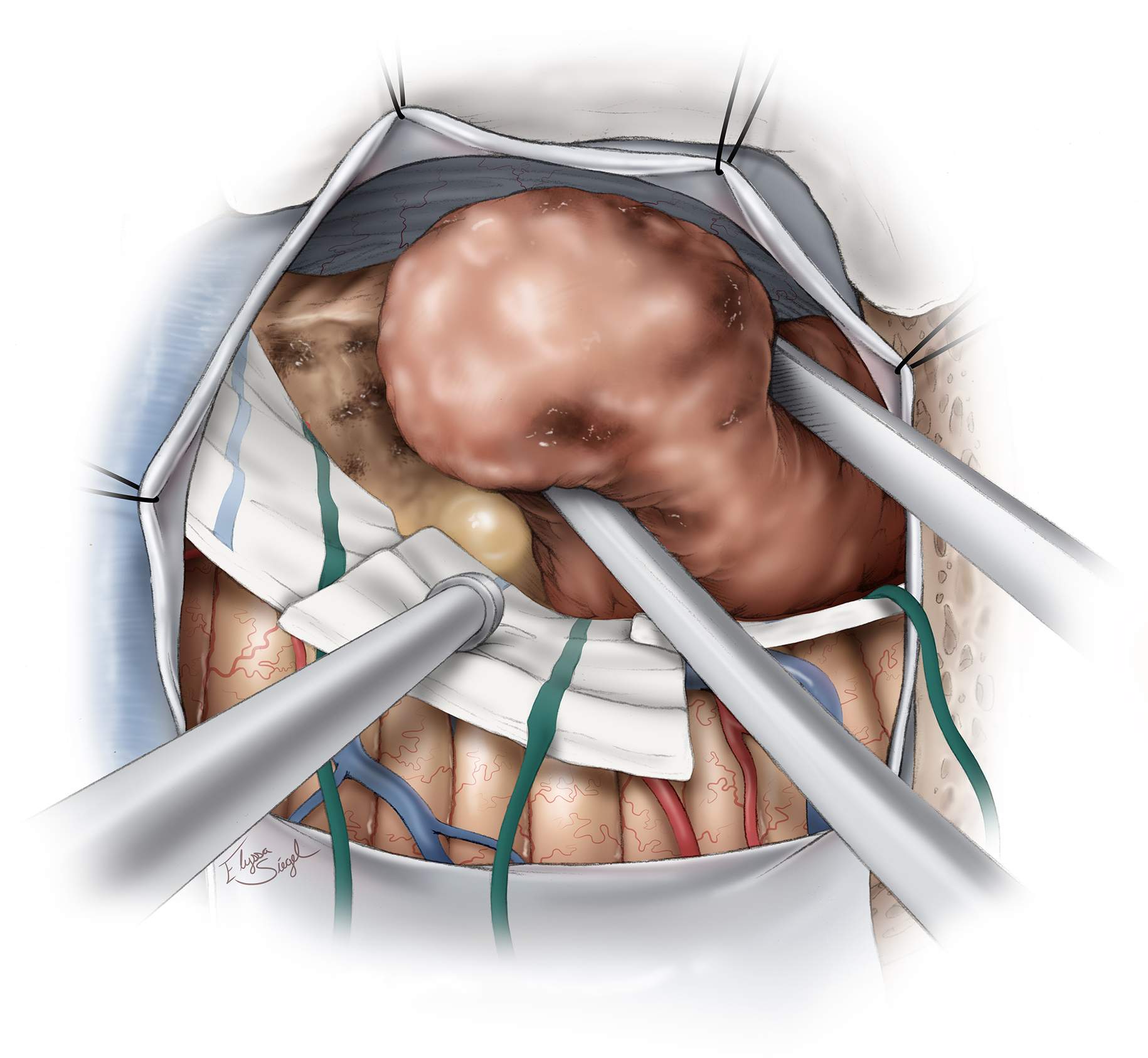
Figure 7: Once the feeding vessels are circumferentially disconnected, the draining veins are also sacrificed. The gliotic plane between the white matter and the nodule is entered. The deep white matter feeders that are present in AVMs are not present in hemangioblastomas. This fact simplifies hemangioblastoma surgery tremendously. Finally, the nodule is delivered en bloc.
Figure 8: The cyst wall does not require removal for cure. Once the nodule is removed, the cyst walls are explored to confirm that the nodule is completely excised. Immaculate hemostasis is secured.
Large Solid Brainstem Hemangioblastomas
Solid hemangioblastomas require a more nuanced approach; they act like an entirely different set of highly vascular lesions that belong to the AVM category. The tumor is comprised of a collection of capillaries that bleed easily and are not amenable to bipolar coagulation. This feature makes their resection risky. Piecemeal resection of them should not be attempted.
Absolute hemostasis is critical during the operation to avoid life-threatening blood loss. It is important for the arterial feeders to be initially accessed and immediately interrupted. This maneuver may not always be feasible with brainstem lesions, especially if medial dural and pial feeders are involved. Preoperative embolization is therefore strongly recommended. Cerebellar swelling is not unlikely with interruption of the vessels associated with these lesions, and a staged approach is not only reasonable but also advised for giant hemangioblastomas.
There are often large draining veins on the surface of the nodule. The mere gentle manipulation of the veins leads to their avulsion and massive bleeding. Their control can be challenging and their sacrifice leads to hemodynamic changes within the hemangioblastoma that can precipitate more bleeding. This vicious cycle is most likely the mechanism that makes these lesions highly risky to handle. I therefore re-emphasize the need for aggressive devascularization through preoperative embolization and early intraoperative disconnection of the feeding arteries. Strategic maneuvers are necessary.
Piecemeal removal of the lesion to create more space to work through and around the vital brainstem structures is not feasible. Lesional deflation through interruption of the feeding vessels is the most reasonable plan.
Giant Recurrent Cerebellar Hemangiopericytoma
Large Solid Brainstem Hemangioblastomas: Daunting Challenges
Recurrent Cerebellar Hemangioblastoma
Postoperative Considerations
Because of the high vascularity of these tumors, an intracerebral hematoma can develop with uncontrolled postoperative hypertension. The patient is observed in the intensive care unit for one or two days after surgery before being transferred to the ward.
Pearls and Pitfalls
- Resection of large solid brainstem hemangioblastomas can be among the most challenging procedures in neurosurgery. These lesions should be treated like high-flow AVM lesions and undergo preoperative embolization.
Contributor: Richard Kim, MD
References
Rengachary SS and Suskind DL. Hemangioblastomas. In Apuzzo MLJ. Brain Surgery: Complication Avoidance and Management, Volume 2. Churchill Livingstone, 1825-33, 1983.
Please login to post a comment.