Anaplastic Astrocytoma
Figure 1: (Top Left) Axial FLAIR demonstrates a left insular infiltrative lesion involving the cortex and white matter. This anaplastic astrocytoma is fairly circumscribed, a less common characteristic in these higher grade lesions. (Top Right) ADC demonstrates no appreciable dark restricted diffusion in this lesion to suggest hypercellularity. (Bottom) Axial T1WI postcontrast shows a small round area of enhancement in the superficial insula, a feature more typical of higher grade glioma. If the T2/FLAIR hyperintensity surrounding this lesion spared the cortex, the enhancing lesion may be more easily mistaken for metastasis with surrounding vasogenic edema.
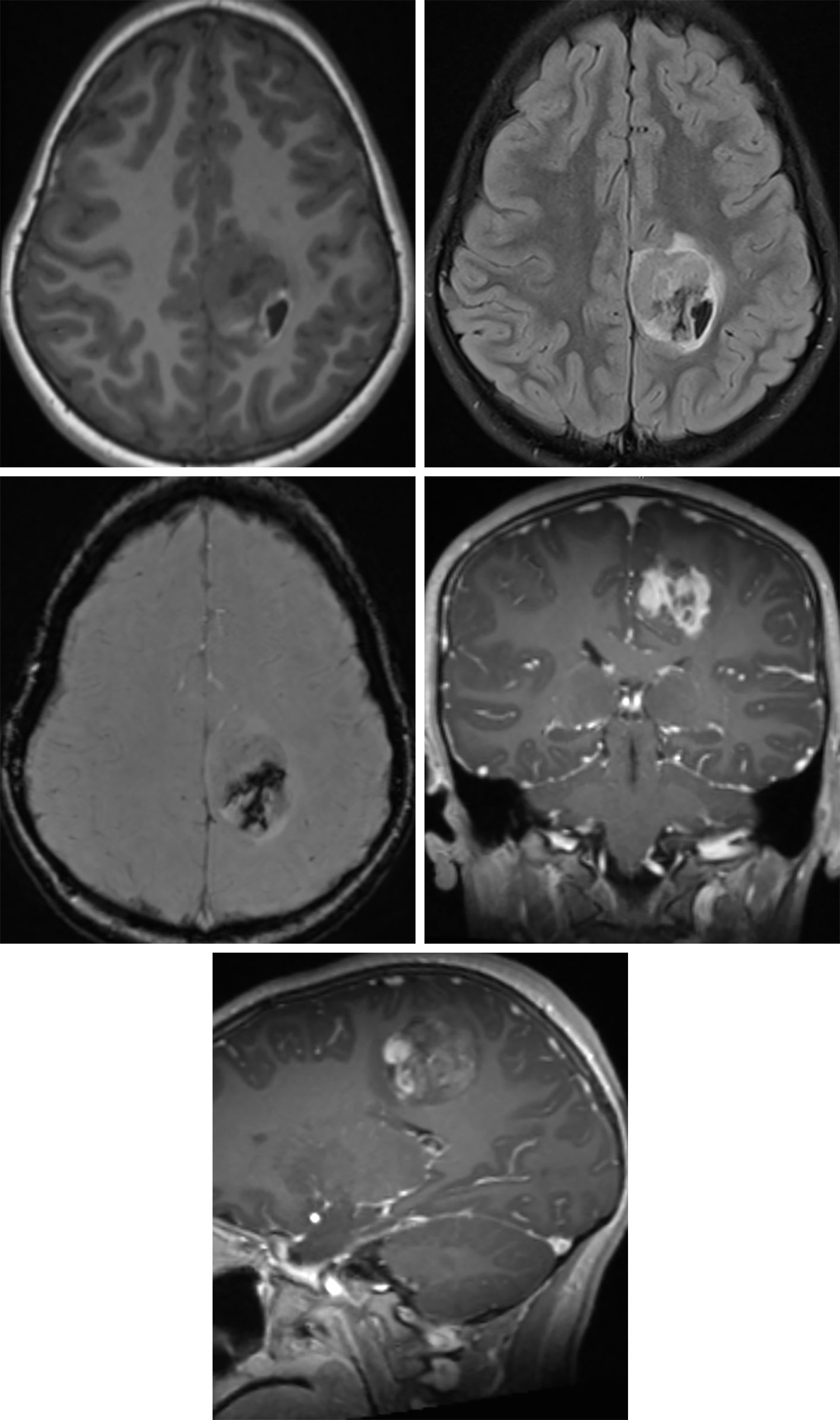
Figure 2: The medial left frontoparietal complex-appearing anaplastic astrocytoma in this patient demonstrates low T1 signal intensity (top left) and FLAIR hyperintensity (top right) with a mixed-signal hemorrhagic component at its posterolateral aspect. Black signal on the susceptibility-weighted imaging (middle left) also reflect this hemorrhage. The tumor also has a heterogeneous enhancement pattern (middle right, coronal; bottom row, sagittal) that is more typical of higher-grade adult primary brain tumors than of grade II tumors.
BASIC DESCRIPTION
- Infiltrating malignant astrocytoma with ill-defined tumor margins and extensive edema
PATHOLOGY
- WHO grade III
- Usually develops from malignant degeneration of low-grade astrocytoma (WHO grade II)
- Commonly dedifferentiates into glioblastoma (GBM) (~50%) within 2 years
- Focal or diffuse anaplasia, highly proliferative
- Increased cellularity and nuclear atypia; usually no necrosis or microvascular proliferation
CLINICAL FEATURES
All ages affected (fourth and fifth decades of life most common)
- Slight male gender predilection
- Median survival 2–3 years
- Better prognosis with younger age, gross total resection, absence of enhancement, KI-67 index ≤5.1%, and IDH1- or MGMT-positive genetics
- Presenting symptoms dependent on tumor location
- Seizures, headaches, behavioral changes, clinical deterioration in patients with known low-grade astrocytoma
- Treatment: resection, chemotherapy (temozolomide), radiation
IMAGING
- General
- Ill-defined, infiltrating white matter mass
- Location in frontal and temporal lobes most common; brainstem and spinal cord uncommon
- Infiltrates beyond apparent imaging tumor margins
- Expansion and involvement of adjacent cortex common
- Variable enhancement
- Usually nonenhancing, but patchy or nodular enhancement may be present
- Cysts and hemorrhage are uncommon features
- Spreads along white matter tracts, but may spread via cerebrospinal fluid (CSF), leptomeninges, and ependyma
- Ill-defined, infiltrating white matter mass
- CT
- Hypodense, ill-defined white matter mass
- Hemorrhage and calcification uncommon features
- Usually does not enhance on contrast-enhanced CT, but may show focal or patchy enhancement
- Ring enhancement suggests progression to GBM
- MRI
- T1WI: isointense to hypointense
- T2WI: heterogeneously hyperintense; presence of flow voids suggests vascular proliferation and progression to GBM
- FLAIR: heterogeneously hyperintense
- DWI: usually no diffusion restriction
- T1WI+C: usually no enhancement; may show patchy or nodular enhancement
- MRS/MR perfusion: decreased NAA, increased Cho/Cr ratio, increased relative cerebral blood volume (rCBV) compared with low-grade astrocytomas, although usually less than GBMs
- Diffusion tensor imaging (DTI) may assist in surgical planning
IMAGING RECOMMENDATIONS
- MRI with contrast; MR spectroscopy (MRS), MR perfusion, functional MRI, and DTI are useful adjuncts
For more information, please see the corresponding chapter in Radiopaedia.
Contributor: Rachel Seltman, MD
References
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109. doi.org/10.1007/s00401-007-0243-4.
Nayak L, Panageas KS, Reiner AS, et al. Radiotherapy and temozolomide for anaplastic astrocytic gliomas. J Neurooncol 2015;123:129–134. doi.org/10.1007/s11060-015-1771-8.
Ogura R, Tsukamoto Y, Natsumeda M, et al. Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology 2015;35:324–335. doi.org/10.1111/neup.12196.
Osborn AG, Salzman KL, Jhaveri, MD. Diagnostic Imaging (3rd ed). Elsevier, Philadelpha, PA; 2016.
Arevalo-Perez J, Peck KK, Young RJ, et al. Dynamic contrast-enhanced perfusion MRI and diffusion-weighted imaging in grading of gliomas. J Neuroimaging 2015;25:792–798. doi.org/10.1111/jon.12239.
Gempt J, Soehngen E, Förster S, et al. Multimodal imaging in cerebral gliomas and its neuropathological correlation. Eur J Radiol 2014;83:829–834. doi.org/10.1016/j.ejrad.2014.02.006.
Hirai T, Murakami R, Nakamura H, et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol 2008;29:1505–1510. doi.org/10.3174/ajnr.A1121.
Tortosa A, Viñolas N, Villà S, et al. Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer 2003;97:1063–1071. doi.org/10.1002/cncr.11120.
Please login to post a comment.