Meniere’s Disease
This is a preview. Check to see if you have access to the full video. Check access
Vestibular neurotomy for Meniere's disease: Technique
Meniere’s disease is characterized by episodic vertigo, fluctuating hearing loss for low frequencies, and tinnitus. Diagnostic criteria for the condition have been established by the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) based on the initial characteristics of the disease set forth by Prosper Meniere in the 19th century.
Meniere’s is a purely clinical diagnosis, and the guidelines set by the AAO-HNS can tailor the diagnosis to range from “possible” to “certain” based on a number of criteria. Criteria for “certain” Meniere’s include two or more definite and spontaneous episodes of vertigo lasting longer than 20 minutes, fluctuating hearing loss documented by pure tone audiometry, tinnitus or fullness in the affected ear, and histopathologic confirmation of endolymphatic hydrops.
The vagaries in the criteria and the overlap of symptoms with other disorders create a challenge for the clinician attempting to establish a reliable diagnosis of Meniere’s on clinical grounds alone. Other conditions that can mimic Meniere’s disease include benign positional vertigo, vestibular neuritis, and labyrinthitis.
Pathophysiologically, Meniere’s disease results from distension of the endolymphatic spaces or endolymphatic hydrops. Fibrosis of the labyrinth can occur in chronic cases. When the potassium-rich endolymph leaks into the potassium-deprived perilymph, vertiginous attacks appear due to depolarization of the vestibular nerve endings. Many factors may contribute to the development of hydrops of the endolymphatic sac, including increased endolymph production and decreased endolymph absorption, ionic imbalance, genetic abnormalities, viral infection, autonomic imbalance, dietary factors, autoimmune reactions, vascular irregularities, and allergic responses.
The diagnostic workup for Meniere’s primarily focuses on eliminating other causes for the patient’s symptoms. Baseline pure tone audiogram scores should be obtained for every patient who has symptoms of vestibular dysfunction. These can be followed over time to assess fluctuations in hearing function, especially sensorineural hearing loss that preferentially affects the lower frequencies.
In addition, speech discrimination scores, tympanometry, and electronystagmography should be obtained. Imaging studies such as magnetic resonance imaging (MRI) can rule out other causes of Meniere’s disease such as neoplastic or demyelinating disorders. Blood tests may be performed to rule out vasculitic conditions. Our otolaryngologist colleagues are often responsible for patient selection and the initial medical and surgical therapy for these patients.
The initial treatment for Meniere’s follows a two-pronged approach: control of acute symptoms and prevention of further attacks. Acute attacks of Meniere’s, which consist of vertigo, tinnitus, and hearing fluctuations, can be treated with vestibular suppressants such as meclizine or promethazine. Patients presenting to the emergency department are often treated with intravenous fluids. Interventions to help reduce the frequency of attacks are not very effective and include salt restriction and diuretic therapy.
Approximately 10% of patients are refractory to best medical therapy and might benefit from ablative interventions. One such intervention is intratympanic injection of gentamicin, an ototoxic agent that has been shown to block cell signaling in the hair cells of the inner ear. A recent meta-analysis found that approximately 75% and 93% of patients experience complete or substantial control, respectively, of the vertiginous symptoms with this therapy.
Patients who do not benefit from these therapies are considered candidates for a number of surgical options. Patient selection is absolutely crucial, as with surgery for trigeminal neuralgia. Patients without classic, specific symptoms are unlikely to benefit from surgical intervention.
The initial surgical techniques for treatment of Meniere’s disease include the following: 1) endolymphatic sac shunting, which allows decompression of the endolymphatic sac by its drainage into the subarachnoid or the perilymphatic space; and 2) labyrinthotomy, a relatively minor procedure that can be performed under general anesthesia. Labyrinthotomy disrupts the cochlear duct and spiral lamina and thus disrupts hearing as well. The more commonly performed labyrinthectomy can also treat Meniere’s disease; in this procedure the lateral semicircular canal is opened and the vestibule is drilled away. This procedure naturally disrupts hearing function.
Vestibular neurotomy or neurectomy is now the most common neurosurgical procedure for relieving intractable Meniere’s disease, and it has been performed through a translabyrinthine, retrolabyrinthine, or retrosigmoid route.
The best approach used to section the vestibular nerve remains a matter of debate. Historically, in 1961, House described the middle cranial fossa approach to section the inferior and superior vestibular nerves, but this approach has been abandoned because of the heightened risk of deafness and facial weakness.
The retrolabyrinthine approach was described by Norrel and Silverstein in 1978 and modified by Silverstein in 1986 to include only the retrosigmoid approach.
Diagnosis and Evaluation
The American Academy of Otolaryngology–Head and Neck Surgery has established specific guidelines for diagnosis of Meniere’s disease. Based on the number of clinical and histopathologic features present, the diagnosis of Meniere’s disease is classified into certain, definitive, probable, or possible. A “certain” diagnosis requires a histopathologic diagnosis, and most patients fall into the “definitive” category. A “definitive” diagnosis of Meniere’s disease requires two or more spontaneous vertiginous episodes of 20 minutes or longer and audiometrically documented hearing loss on one occasion, tinnitus or aural fullness in the affected ear, and all other causes excluded.
Patients should be queried about the nature of vertigo, subjective feeling of hearing loss and aural fullness, precipitating factors for the onset of vertiginous episodes, prior history of ear surgeries, trauma, and use of ototoxic medications. It is very important to differentiate between Meniere’s disease and benign paroxysmal positional vertigo. There is no hearing loss evident with the latter.
Any focal finding on the neurologic exam suggests a central lesion that needs to be excluded by imaging. If the patient is suffering from nystagmus, the physician should attempt to differentiate between central and peripheral nystagmus. Detailed otologic and vestibular exams are performed by our otolaryngologist colleagues and are beyond the scope of this text.
No gold standard test exists at this time that can reliably diagnose Meneire’s disease with satisfactory sensitivity and specificity. However, the treating physician should be able to rule out metabolic, infectious, and hormonal abnormalities. The patient’s urine should be tested for proteinuria and hematuria to rule out oto-renal syndrome. Neurosyphilis and Lyme disease may be excluded by checking Venereal Disease Research Laboratory (VDRL) tests and a fluorescent treponemal antibody test; symptoms for these conditions can closely mimic Meniere’s disease.
Audiologic testing by audiometry is a first-line investigation to establish the diagnosis. Classically, the hearing loss in Meniere’s disease is described as “fluctuating, low-frequency sensorineural” loss that might progress if the underlying problem is not promptly addressed. Electronystagmography (ENG) may be performed after a 2-week holiday from any vestibular suppressant and includes oculomotor evaluation as well as caloric and positional tests.
Electrocochleography (ECoG) is another diagnostic tool that measures the ratio of the summating potential to action potential in response to an auditory stimulus. A ratio of greater than 35% during an active episode is indicative of Meniere’s disease. Recently new techniques have emerged that may help the physician reach a correct diagnosis. These include vestibular-evoked myogenic potential (VEMP), cochlear hydrops analysis masking procedures (CHAMPs), and 3D FLAIR sequence MRI obtained 24 hours after an intratympanic injection of gadolinium.
A long list of differential pathologies closely mimic Meniere’s disease. Acoustic neuroma, endolymphatic sac tumor, multiple sclerosis, and transient ischemic attacks can be ruled out with the help of MRI. Migraine-associated vertigo is a consideration among patients with a history of migraine headaches. Even diabetes and thyroid disease can cause symptoms that overlap with those of Meniere’s disease. Cogan's syndrome is a chronic inflammatory condition in young adults that has vestibuloauditory symptoms similar to those of Meniere’s disease, but patients with this condition manifest eye involvement and systemic vasculitis.
Indications for Surgery
The goals of any treatment for Meniere’s disease are to reduce the frequency and severity of vertigo attacks and hearing loss while preventing disease progression. The first steps in treatment include lifestyle adjustments such as managing stress, and avoiding high sodium intake, caffeine, alcohol, nicotine, and monosodium glutamate. Diuretics and betahistine may reduce endolymphatic hydrops and improve symptoms.
Approximately 10% of patients with refractory symptoms are eligible for one of the nonablative or ablative surgical procedures. Nonablative procedures include endolymphatic sac procedures (enhancement or shunting) and sacculotomy, and are associated with a low risk of sensorineural hearing loss. Ablative procedures include intratympanic gentamicin, labyrinthectomy, and vestibular neurotomy. Vestibular neurotomy offers symptomatic relief in up to 95% of appropriately selected patients. The procedure is associated with a low risk of sensorineural hearing loss and is one of the safest and most effective procedures to treat Meniere’s disease.
The following offering describes my experience with vestibular nerve section (neurotomy) through a retromastoid craniotomy.
Preoperative Considerations
All patients with a diagnosis of Meniere’s disease should undergo audiometric testing and MRI before surgery. As my experience has grown, I have abandoned the practice of routinely monitoring the facial nerve intraoperatively. However, I do routinely monitor brainstem auditory evoked responses (BAERs) to minimize the risk of postoperative hearing loss. This modality alerts me to adjust dynamic retraction during manipulation of the cranial nerve (CN) VII/VIII complex.
Operative Anatomy
Click here to view the interactive module and related content for this image.
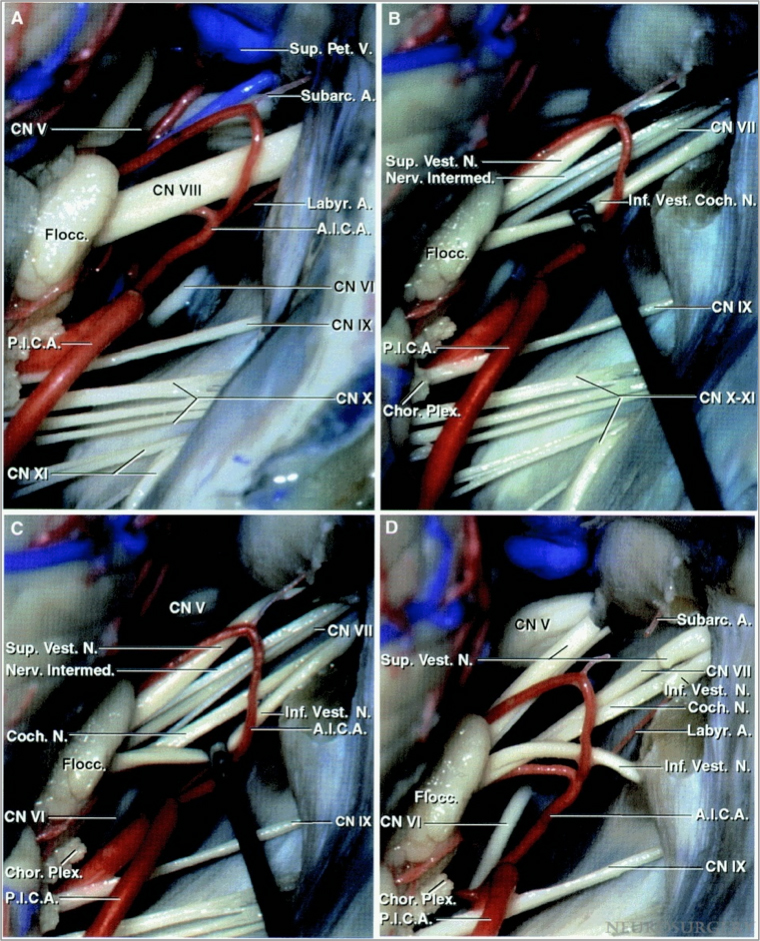
Figure 1: Exposure of the cranial nerves within the cerebellopontine angle after a right-sided retrosigmoid approach. Note the relationship of the anterior inferior cerebellar artery (AICA) and the posterior inferior cerebellar artery (PICA) to the CN VII/VIII complex (A). A clear understanding of the relationship of the nerves within the CN VII/VIII complex is important. The facial nerve is located anterior to the superior vestibular nerve (B).
A cleavage plane developed between the cochlear nerve and the inferior vestibular nerve (C). The superior and inferior vestibular nerves were divided to expose the facial and cochlear nerves (D). A labyrinthine branch of the AICA is also apparent (Images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 2: These images demonstrate the location of the vestibular nerves in relationship to the facial and cochlear nerves. Note the specific location of the cochlear nerve along the inferior aspect of CN VIII (Images courtesy of AL Rhoton, Jr).
VESTIBULAR NEUROTOMY
The retromastoid craniotomy for vestibular nerve section is the same as the one for microvascular decompression for trigeminal neuralgia. I use the supralateral cerebellar approach to expose the CN VII/VIII complex.
Please refer to the chapter on extended retromastoid craniotomy for a detailed description of the approach to CN VIII.
Figure 3: The operative corridors and trajectories for accessing the cerebellopontine angle: The surgical corridors for microvascular decompression for trigeminal neuralgia (supralateral cerebellar approach-blue arrow) and hemifacial spasm, and glossopharyngeal neuralgia (infralateral cerebellar or infrafloccular approach-green arrow) are illustrated. I use the supralateral cerebellar corridor to access CN VIII.
INTRADURAL PROCEDURE
A piece of glove (cut slightly larger than the cottonoid patty) acts as a rubber dam. It protects the cerebellar hemisphere against the rough surface of the cottonoid as the rubber dam slides over the cerebellum while dissection is continued to expose the cerebellopontine angle. I identify the junction of the petrous bone and tentorium and advance the cottonoid over the rubber dam near the turn of the petrous bone toward the lower aspect of CN V and upper aspect of CN VII/VIII.
Medial retraction of the cerebellum parallel to CN VII/VIII is avoided to prevent direct transmission of retraction to these nerves. The alternating vectors of retraction are parallel to CN’s V and IX. Note that I do not apply fixed retractors, but instead use the suction apparatus to mobilize the cerebellar hemisphere in a dynamic fashion during dissection. Along with generous opening of the regional arachnoid membranes over the cranial nerves, this maneuver minimizes the risk of hearing loss. Dynamic retraction of the suction apparatus allows intermittent exposure only where needed. Aggressive retraction of fixed retractors often provides exposure at places that may not be necessary.
Figure 4: Exposure of the right CN VII/VIII complex through the right-sided retromastoid approach. The vestibular component of the nerve is usually more grayish in color than the cochlear component. An arteriole often delineates the demarcation line along these two components. Intraoperative BAER monitoring guides the surgeon to adjust cerebellar retraction to minimize undue traction on CN VIII.
I continue irrigating the field periodically during intradural dissection because the intense light of the microscope can cause heat injury to CN VIII. I may also cover the inferior surface of CN VIII with a small piece of papaverine-soaked gelfoam to relieve vasospasm. If the BAERs change at anytime during the intradual maneuvers, I perform the following steps:
- Stop dissection and relieve all retraction while irrigating the operative field.
- Allow a few minutes for the BAERs to return to normal. It may be necessary to increase the blood pressure. Before reapplying dynamic retraction, I further dissect the arachnoid membranes over CN VII/VIII to relieve any traction on these nerves while mobilizing the medial cerebellum. I also cover these nerves with a small piece of papaverine-soaked sponge to relieve any vasospasm caused by traction and the heat of the microscope.
- I then attempt a more superior-to-inferior directed retraction while minimizing any traction parallel to the CN VII/VIII complex.
The facial nerve is usually concealed anteriorly, and it is generally best to avoid its dissection. The arachnoid membranes over the complex can be reflected medially. This maneuver allows better visualization of the plane between the two components of CN VIII, often better appreciated closer to the porus acusticus. It is a good practice to confirm the identity of the cranial nerve by angling the microscope to view up toward CN V and down toward CN’s IX and X.
Some surgeons prefer to drill into the internal auditory canal to expose the complex more laterally and delineate the plane between the vestibular and cochlear branches. I have not used this technique because in most patients, the delineation between the branches of CN VIII is sufficiently apparent more laterally along their intracisternal segment, making drilling into the internal auditory canal unnecessary. It is important to take great care to avoid excessive manipulation of the nerve complex and its associated vasculature to avoid injury to the cochlear nerve and its blood supply.
Figure 5: Accessing the cleavage plane by using a blunt angled microdissector to separate the vestibular and cochlear divisions. A reasonable plane can often be found.
Figure 6: Once the plane within the nerve is identified, the vestibular portion is sectioned with microscissors. The edges of the cut nerve retract and CN VII comes into view anteriorly. While sectioning the vestibular nerve, I direct the tip of the microscissors superiorly to avoid damaging the ventrally located facial nerve. Some operators advocate removing a portion of the vestibular nerve to avoid possible reinnervation.
Figure 7: I then use a sickle knife to ensure that all the fibers of the vestibular nerve are transected. Once the vestibular neurotomy is complete, the labyrinthine artery may become visible. Excessive manipulation of this important artery should be avoided to preserve hearing.
Figure 8: A relatively easily identifiable nerve sheath often encases the inferior portion of CN VIII. The facial nerve is exposed along the anterior aspect of the neurotomy.
Closure
The dura is approximated primarily. I do not perform a watertight dural closure and have experienced very low rates of cerebrospinal fluid leak through both the incision and the nose. Mastoid air cells are rewaxed thoroughly (“wax in, wax out”) and the bone flap is replaced or a methyl methacrylate cranioplasty is performed. The muscle and scalp are closed in anatomic layers.
Further details regarding closure may be found in the dedicated chapter related to retromastoid craniotomy.
Postoperative Considerations
Patients are usually admitted to the Neuro ICU overnight for observation and then transferred to the regular ward for a couple of days before they are discharged home. Special attention should be paid to hemodynamic parameters, the neurologic examination, and wound care. Steroids are administered to prevent aseptic meningitis and minimize postoperative nausea and headaches. As with any retrosigmoid craniotomy, the patient should be closely monitored for any sign of cerebrospinal fluid leakage.
Hearing loss is a real risk of this operation because the cochlear nerve is manipulated. A deficit will be immediately evident after the surgery. Any patient with subjective hearing loss should undergo pure tone audiometry postoperatively. Brainstem auditory evoked response monitoring during surgery can help reduce the incidence of postoperative hearing loss. Reported rates of hearing loss are in the 5-10% range.
Particular to this operation, the patient may develop a sense of vertigo, severe nausea, and/or nystagmus. This is related to the acute loss of input from one of the vestibular organs and its sudden deafferentation; these symptoms can be treated with antiemetics and vestibular suppressants. These symptoms are temporary and resolve within 1-2 weeks. Some patients may benefit from vestibular rehabilitation therapy. Vestibular rehabilitation uses exercise activities to maximize balance and central nervous system compensation for disequilibrium.
The preoperative vertiginous symptoms of most patients (~90%) improve significantly after surgery, and many are free of attacks. Unfortunately, no large trials have been conducted to establish the efficacy of this surgery, and current literature consists of small case series. However, almost all patients who undergo the surgery report improved quality of life and many are able to return to work. Therefore, the procedure is indeed beneficial when performed on a properly selected patient.
Pearls and Pitfalls
- Meniere’s disease is a disorder of the endolymphatic hydrops characterized by vertiginous attacks, low frequency sensorineural hearing loss, tinnitus, and aural fullness.
- Correct diagnosis can be difficult but is paramount for satisfactory operative results. Accurate diagnosis requires a thorough history and physical examination along with dedicated otolaryngological tests.
- Lifestyle and dietary modifications, diuretics, and vestibular sedatives are the first line treatments. Patients with refractory symptoms can be considered for more invasive procedures.
- Vestibular neurotomy is performed through a retromastoid approach and carries a low risk of hearing loss and facial palsy.
- During sectioning of the vestibular nerve, the cutting direction should be as parallel to the width of CN VII as possible to avoid facial nerve injury. The labyrinthine artery should not be manipulated to avoid its vasospasm and subsequent hearing loss.
- Due to sudden deafferentation of the vestibular organs on the operated side, patients may develop a sense of vertigo, nausea, and/or nystagmus postoperatively. These symptoms are usually self-limiting and resolve over 1-2 weeks.
For additional illustrations of vestibular neurectomy, please refer to the Jackler Atlas by clicking on the image below:
Contributor: Aqueel Pabaney, MD
References
American Academy of Otolaryngology-Head and Neck Foundation Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. Otolaryngol Head Neck Surg 1995;113:181-185.
Cohen-Kerem R, Kisilevsky V, Einarson TR, Kozer E, Koren G, Rutka JA. Intratympanic gentamicin for Menière's disease: A meta-analysis. Laryngoscope 2004;114:2085-2091.
Greenberg SL, Nedzelski JM. Medical and noninvasive therapy for Meniere's disease. Otolaryngol Clin North Am 2010;43:1081-1090.
Le CH, Truong AQ, Diaz RC. Novel techniques for the diagnosis of Ménière's disease. Curr Opin Otolaryngol Head Neck Surg 2013;21:492-496.
Li CS, Lai JT. Evaluation of retrosigmoid vestibular neurectomy for intractable vertigo in Ménière's disease: An interdisciplinary review. Acta Neurochir (Wien) 2008;150:655-661
Magnan J, Bremond G, Chays A, Gignac D, Florence A. Vestibular neurotomy by retrosigmoid approach: Technique, indications, and results. Am J Otol 1991;12:101-104.
Schlegel M, Vibert D, Ott SR, Haeusler R, Caversaccio MD. Functional results and quality of life after retrosigmoid vestibular neurectomy in patients with Meniere's disease. Otol Neurotol 2012;33:1380-1385.
Stapleton E, Mills R. Clinical diagnosis of Ménière's disease: How useful are the American Academy of Otolaryngology Head and Neck Surgery Committee on Hearing and Equilibrium guidelines? J Laryngol Otol 2008;122:773-779.
Syed I, Aldren C. Meniere's disease: An evidence based approach to assessment and management. Int J Clin Pract 2012;66:166-170.
Tarlov EC, Poe DS. Selective microsurgical rhizotomy for intractable Meniere's syndrome. In: Apuzzo M, (ed) Brain Surgery: Complication Avoidance and Management, 1st ed. New York: Churchill Livingstone; 1992, 2145-2151
Teufert KB, Doherty J. Endolymphatic sac shunt, labyrinthectomy, and vestibular nerve section in Meniere's disease. Otolaryngol Clin North Am 2010;43:1091-1111.
Perez R, Ducati A, Garbossa D, Benech F, Fontanella MM, Canale A, Albera R. Retrosigmoid approach for vestibular neurectomy in Meniere's disease. Acta Neurochir (Wien) 2005;147:401-404
Schlegel M, Vibert D, Ott SR, Haeusler R, Caversaccio MD. Functional results and quality of life after retrosigmoid vestibular neurectomy in patients with Meniere's Ddisease. Otol Neurotol 2012;33:1380-1385.
Please login to post a comment.