Diagnosis and Evaluation of Aneurysmal Subarachnoid Hemorrhage
Subarachnoid hemorrhage (SAH) is defined as bleeding around the brain confined within the subarachnoid space between the arachnoid membrane and the pia mater. The etiology of SAH can be divided into two main categories: 1) spontaneous, and 2) traumatic. I will discuss only spontaneous SAH in this chapter.
The incidence of aneurysmal SAH reaches an annual rate of 6 to 8 per 100,000. Its sequelae can lead to death and significant functional disability, with mortality rates reaching 45%. A number of causes are associated with development of spontaneous SAH.
| Ruptured intracranial aneurysm (75-89%) |
| Cerebral arteriovenous malformation |
| Dural and pial arteriovenous fistula |
| Dural venous sinus thrombosis |
| Pretruncal/perimesencephalic nonaneurysmal SAH |
| Cerebral artery dissection (internal carotid and vertebral arteries) |
| Rupture of an infundibulum |
| Pituitary apoplexy |
| Coagulation disorder (bleeding dyscrasias and thrombocytopenia) |
| CNS vasculitis |
| Brain tumor |
| Spinal arteriovenous malformation (cervical or high thoracic) |
| Unknown or idiopathic |
Timely diagnosis and focused management of spontaneous SAH, therefore, are paramount to prevent rehemorrhage and the secondary effects of SAH. Better outcomes are achieved at high-volume neurovascular centers.
Presentation
A thunderclap headache refers to a severe headache with its peak intensity at onset, thus as sudden and unexpected as a clap of thunder. Patients with the classic sudden thunderclap headache or “worst headache of their life” are considered to be suffering from spontaneous SAH until proven otherwise.
This aggressive stance promotes a high index of suspicion, timely and efficient assessment, minimizes misdiagnosis, and potentially leads to effective management. The term thunderclap headache has also been used to refer to an idiopathic benign recurrent headache disorder (migraine variant). In fact, the differential diagnosis of a sudden-onset headache can be broad. It includes SAH, cerebral venous thrombosis, pituitary apoplexy, spontaneous intracranial hypotension, and hypertensive encephalopathy.
One might wonder why all of these seemingly disparate conditions can bring about similar clinical manifestations. Any etiology leading to a sudden change in the pressure within the subarachnoid space causes two sequelae:
- transmission of pressure to the rest of the intradural space, thereby suddenly increasing the outward force on the dura (causing headache), and
- thereby decreasing the cerebral perfusion pressure (leading to loss of consciousness, confusion, and/or delayed ischemic neurologic deficit).
The initial diagnostic workup includes a detailed history and physical exam. The history should focus on questions that relate to the patient’s characterization of the headache and associated symptoms: worst headache of their life, rapid onset, meningismus, loss of consciousness, nausea, vomiting, photophobia, phonophobia, diplopia, focal neurologic deficit, back pain, seizure, weakness, presence or absence of sentinel headaches, and association with another activity (e.g., cocaine use and sexual activity).
The most significant modifiable risk factors for aneurysmal SAH are hypertension and cigarette smoking. Other modifiable risk factors include heavy alcohol use and illicit drug abuse (especially sympathomimetics: cocaine and methamphetamines).
Nonmodifiable risk factors include sex (females), race (African-American), personal or family history of cerebral aneurysm, and previous history of SAH, stroke, or cerebrovascular disease. Nonaneurysmal SAH may incorporate some if not all of the factors listed above. Therefore, the diagnostic workup for SAH, regardless of its etiology, needs to proceed in the same manner for each occurrence of a thunderclap headache.
If history, physical exam, and diagnostic imaging point to the diagnosis of SAH, then the root cause of SAH must also be carefully evaluated. Ruptured intracranial aneurysms account for the largest proportion of patients presenting with spontaneous SAH.
The symptomatology should lead to swift imaging (a computed tomography (CT) scan) that will serve as an initial diagnostic aid to determine the presence of subarachnoid blood. Timely exclusion of aneurysmal SAH is crucial to the patient’s subsequent management paradigm.
Diagnosis and Evaluation
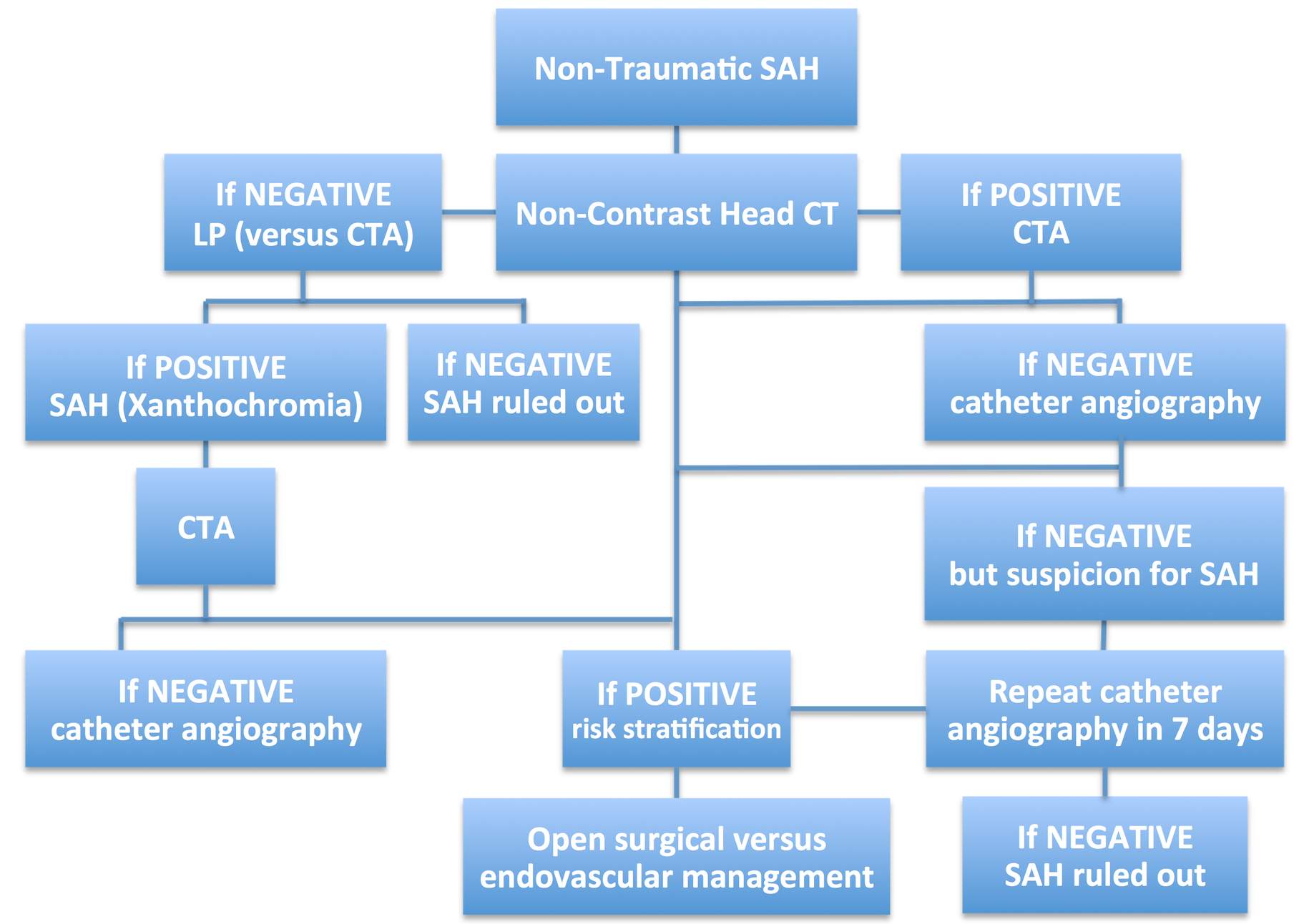
When a patient is suspected to suffer from spontaneous SAH, the initial workup should include a noncontrast head CT. Modern CT scanners have a sensitivity of 98% to detect SAH. These results are reproducible when the scans are obtained within 1 to 5 days after the bleed.
Once the CT scan is positive for SAH, the patient should undergo further imaging to study the vasculature of the head and potentially the neck. The noninvasive vascular study of choice is a CT angiogram (CTA). The CTA can be performed efficiently with minimal risk to the patient (unless the patient has an iodine-related allergy or sensitivity). This modality is very sensitive for detecting the etiology of SAH.
The CTA allows effective assessment of the aneurysm’s morphology, nearby skull base anatomy, and intrasaccular thrombosis. However, CTA suffers from certain disadvantages, as it tends to underestimate the size of the aneurysm; reformatting of the images depends on the expertise of the available technician.
Figure 2: This patient presented with SAH and harbored a large ophthalmic artery aneurysm on initial CTA evaluation. She underwent clip ligation of her aneurysm. Postoperative catheter angiography that was performed for assessment of the adequacy of ophthalmic aneurysm occlusion revealed two ipsilateral small MCA aneurysms (arrows) that were missed on the initial CTA. This finding led to the patient undergoing an unnecessary second surgery.
Figure 3: This patient underwent clip ligation of her ruptured ACoA aneurysm and was found to have a previously undetected (or questionably visible during retrospective review of the initial CTA) ipsilateral MCA aneurysm on postoperative catheter angiography. She subsequently underwent a second surgery for clip ligation of her M1 aneurysm.
The above cases highlighted the potential limitation of the CTA to detect small aneurysms (<4mm), especially within the crowded Sylvian cisterns. These small aneurysms require treatment among patients with a history of SAH from another aneurysm. Their delayed detection on postoperative angiography can lead to unnecessary second surgery.
The bony artifact from the skull base can justify the more frequent use of catheter angiography among patients harboring PCoA and ACoA aneurysms. The relatively increased complexity of the angioarchitecture of the adjacent arterial branches near these aneurysms as compared with those around MCA aneurysms, can explain an increased tendency for these aneurysms to proceed to catheter angiography preoperatively.
CTA has a specificity of 100% and a sensitivity of 96% to 99.7% for aneurysms that are 4 mm or larger. Magnetic resonance imaging (MRI) is another alternative, albeit less efficient and cost effective, for initial screening for detection of blood within the subarachnoid space. Fluid-attenuated inversion recovery (FLAIR) sequences have 100% sensitivity within the first 5 days after SAH, whereas T2-weighted gradient echo sequences have similar sensitivity within 6 to 30 days after the ictus. The sensitivity of magnetic resonance angiography (MRA) for detecting aneurysms >5 mm in size is 85% to 100%.
The practical “real-world” strength of the MRA lies in the outpatient workup of unruptured aneurysms (i.e., when a diagnostic workup is set forth in the absence of intracranial hemorrhage). CTA is superior to MRA for detection and study of the aneurysm’s morphology and its intrasaccular contents. Patients with an iodine-based allergy or sensitivity may undergo MRI/MRA. I use the MRA for screening the patients who may suffer from the familiar forms of cerebral aneurysms.
If the CT scan is unremarkable for SAH or if the headaches occurred days before evaluation, but the patient’s symptoms are suspicious for occurrence of SAH, a lumbar puncture (LP) is indicated. The history and character of the headaches play an important role in the need to proceed with an LP whose purpose is to rule out the presence of xanthochromia.
Xanthochromia is found within hours (most likely after 12 hours) of the ictus and remains within the CSF for 3 to 4 weeks. If the CSF results are positive for xanthochromia, CTA is necessary, and if CTA is unremarkable, a catheter angiogram is indicated.
When the CT and CTA results are unremarkable in a patient suspected of suffering from SAH, there is a 99% likelihood that SAH has been reliably ruled out. The 1% rate of misdiagnosis can be reduced based on effective history taking and performance of an LP.
Pitfalls leading to a misdiagnosis of aneurysmal SAH include failure to recognize the presenting symptoms and to understand the limitations of the CT scan following a sentinel hemorrhage, and failure to perform a lumbar puncture.
Catheter angiography is the gold standard study for evaluation of aneurysms and occult arteriovenous malformations not detectable on other imaging modalities. This from of vascular imaging is unique in its therapeutic abilities via endovascular aneurysm embolization.
Catheter angiography effectively demonstrates the important aneurysm details (aneurysm size, anatomic projection, neck-to-dome ratio, the robustness of the collateral circulation, and the anatomy of the neighboring vascular territories), and unlike CTA, it can provide valuable information about flow dynamics within the corresponding vasculature. In addition, small vessels, dural and skull base vascular anatomic features, and their pathoanatomy can be characterized via multiple projections and three-dimensional (3D) reconstruction modeling.
Most importantly, 3D rotational angiography leads to more sensitive aneurysm detection, provides valuable anatomic information about the aneurysm neck, and allows planning for surgical or endovascular intervention. However, 3D modeling may potentially overestimate the breadth of the aneurysm neck and adherence of the perforating vessels onto the neck and dome.
In order for an angiogram to be considered negative for detection of a cerebral aneurysm, three criteria must be met. The imaging should:
- Study bilateral internal and external carotid arteries, bilateral vertebral arteries, including bilateral posterior inferior cerebellar arteries (this can be performed via a dominant vertebral artery)
- Thoroughly evaluate the anterior communicating artery complex (it may be necessary to perform an internal carotid artery cross compression study)
- Evaluate the presence of an atypical infundibulum near the focus of SAH. This minor vascular abnormality may occasionally require surgical exploration for its exclusion as the source of hemorrhage.
Patients diagnosed with SAH are characterized using reliable grading systems such as the Hunt Hess (HH) and World Federation of Neurological Surgeons (WFNS) grading scales. Based on the presenting HH and WFNS grades, patient outcome data has been extrapolated. For more details regarding these classification systems, please refer to the Vascular Quick Reference Tables.
Overall, CTA and catheter angiograms play complementary rather than competitive roles in the management of complex intracranial aneurysms. CTA is invaluable for detection of calcium or thrombus within the aneurysm neck and dome, respectively. In addition, its analysis of the skull base provides indispensable information about the location of the relevant bony anatomy in relation to the components of the circle of Willis. For example, the anatomy of the anterior and posterior clinoid processes relative to the posterior communicating artery and superior cerebellar/basilar bifurcation aneurysms is important for preoperative planning of the extent of bony removal and selection of the appropriate operative corridor, respectively.
The workup of patients suspected of suffering from nontraumatic SAH is summarized below.
Brief Notes on Management
Important considerations include the patient’s level of consciousness, time of onset of headache (and/or other symptoms) and focal neurologic deficits, as well as the presence of sentinel headaches, seizure, and use of anticoagulation/antiplatelet medications. In patients with an altered mental status, management of the airway, breathing, and circulation must be a priority. Patients who present with a Glasgow coma scale (GCS) score of 8 or worse should be considered for intubation and airway protection.
The patient is admitted to a neuro intensive care unit and appropriate studies are performed, including baseline electrocardiogram (to evaluate for arrhythmias), two-dimensional echocardiography to establish baseline heart function and screen for early cardiac dysfunction secondary to a catecholamine surge prompted by SAH, and relevant laboratory studies. Initial lab tests should include a complete blood count and basic metabolic panel, as well as ionized calcium, magnesium, and phosphorous levels, and prothrombin time, partial thromboplastin time, blood type and screen, arterial blood gas, initial troponin level, and a urine drug screen.
An arterial and central venous catheter may be needed. The arterial line allows close monitoring of blood pressure and frequent blood draws, while the central line measures central venous pressure to assess volume status and determine the etiology of hyponatremia (which is commonly encountered in patients with SAH). Strict blood pressure control is necessary for patients presenting with hypertension and SAH who harbor an unsecured ruptured aneurysm. Typically, systolic blood pressure should be maintained below 140 mm Hg until the aneurysm is treated.
Blood pressure control can be effectively achieved using intravenous administration of a calcium channel blocker such as nicardipine or a beta-blocker such as labetalol. The presence of intraventricular blood and ventriculomegaly/hydrocephalus indicate the need for placement of an external ventricular drain (EVD). The laterality of the EVD should not interfere with the planned side of surgical approach. Liberal drainage of CSF is not advised because this maneuver can cause abrupt changes in intracranial and transmural pressures, leading to aneurysmal rerupture. I usually leave the drain at 15 cm of water rather than the standard 10 cm.
The effects of anticoagulant medications must be reversed. The addition of functional donor platelets is worth considering for patients previously on antiplatelet medications. Anticonvulsant medications, antifibrinolytics, calcium channel blockers, and statins are also administered based on the preference of the surgeon.
Statins are likely to decrease the risks of vasospasm, delayed neurologic deficits, and mortality; they are continued for 30 days. The absence of seizure activity limits the anticonvulsant medication dosing to 7 days. Calcium channel blockers (nimodipine) are administered for 21 days.
After the source of SAH has been established, further effort is focused on securing the ruptured aneurysm. The optimal treatment modality must be addressed on a case-by-case basis. However, there are four main considerations that influence treatment options (microsurgical versus endovascular):
- Aneurysm morphology
- Patient’s age, medical status, and presenting symptoms (e.g., HH grade)
- The preferences of the patient and the family
- The expertise of the treating surgeons/interventionalists
Regardless of the treatment mode, early intervention (within 24 hours of the ictus) has been shown to decrease the mortality rate caused by rehemorrhage. For further details regarding management paradigms, refer to the chapter on Clip or Coil.
Complication management during the posttreatment phase hinges on prevention, detection, and swift management of vasospasm. I routinely request a postoperative catheter angiogram on the ninth posthemorrhage day to confirm complete exclusion of the aneurysm and relief of vasospasm so that the patient to be transferred to the regular ward.
Long-Term Follow-Up after Clip Ligation
The long-term care of patients with microsurgically-treated aneurysms includes a CT or catheter angiogram at the 1-year postoperative visit. If a residual or recurrent aneurysm is not detected and the patient initially harbored only a single aneurysm, long-term surveillance imaging after clip ligation has a questionable utility because of the very low risk of recurrence.
Patients who suffer from multiple aneurysms should continue to undergo imaging every 5 years because of the risk of de novo aneurysm formation. This principle obviously also applies to the patients with residual aneurysms.
Additional Considerations
Patients with an unruptured aneurysm who present with a sentinel headache (a severe nonthunderclap headache that is different from the patient’s usual headaches) should undergo urgent treatment of their aneurysm. These headaches indicate instability of the aneurysm and a pending risk of hemorrhage.
Screening of other family members should be considered if there are first-degree relatives with cerebral aneurysms. Diseases associated with aneurysms, such as coarctation of the aorta, polycystic kidney disease, fibromuscular dysplasia, and sickle cell disease, as well as cocaine use are other potential indications for prophylactic imaging.
Contributor: John A. Braca, III, MD
References
Anzalone N, Triulzi F, Scotti G. Acute subarachnoid hemorrhage: 3D time-of-flight MR angiography versus intra-arterial digital angiography. Neuroradiology. 1995;37:257-261.
Bederson JB, Connolly ES Jr, Batjer HH, et al, American Heart Association. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009; 40:994-1025.
Cortnum S, Sorenson P, Jorgensen J. Determining the sensitivity of computed tomography scanning in early detection of subarachnoid hemorrhage. Neurosurgery. 2010;66:900-902.
Dupont SA, Wijdicks EF, Manno EM, Rabinstein AA. Thunderclap headache and the normal computed tomographic results: Value of cerebrospinal fluid analysis. Mayo Clinic Proc. 2008;83:1326-1331.
Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000; 342:29-36.
Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke. 2005;36:2773-2780.
Sailer AM, Grutters JP, Wildberger JE, Hofman PA, Wilmink JT, van Zwam WH. Cost-effectiveness of CTA, MRA, and DSA in patients with non-traumatic subarachnoid haemorrhage. Insights Imaging. 2013;4:499-507.
van Rooij WJ, Sprengers ME, de Gast AN, Peluso JP, Sluzewski M. 3D rotational angiography: The new gold standard in the detection of additional intracranial aneurysms. Am J Neuroradiol. 2008; 29:976-979.
Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, et al. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis—systematic review and meta-analysis. Radiology. 2011; 258:134-145.
Please login to post a comment.


















Comments: