Temporal AVMs
This is a preview. Check to see if you have access to the full video. Check access
Lateral Temporal AVM: Handling en passage Arteries
Please note the relevant information for patients suffering from arteriovenous malformations is presented in another chapter. Please click here for patient-related content.
Operative Anatomy
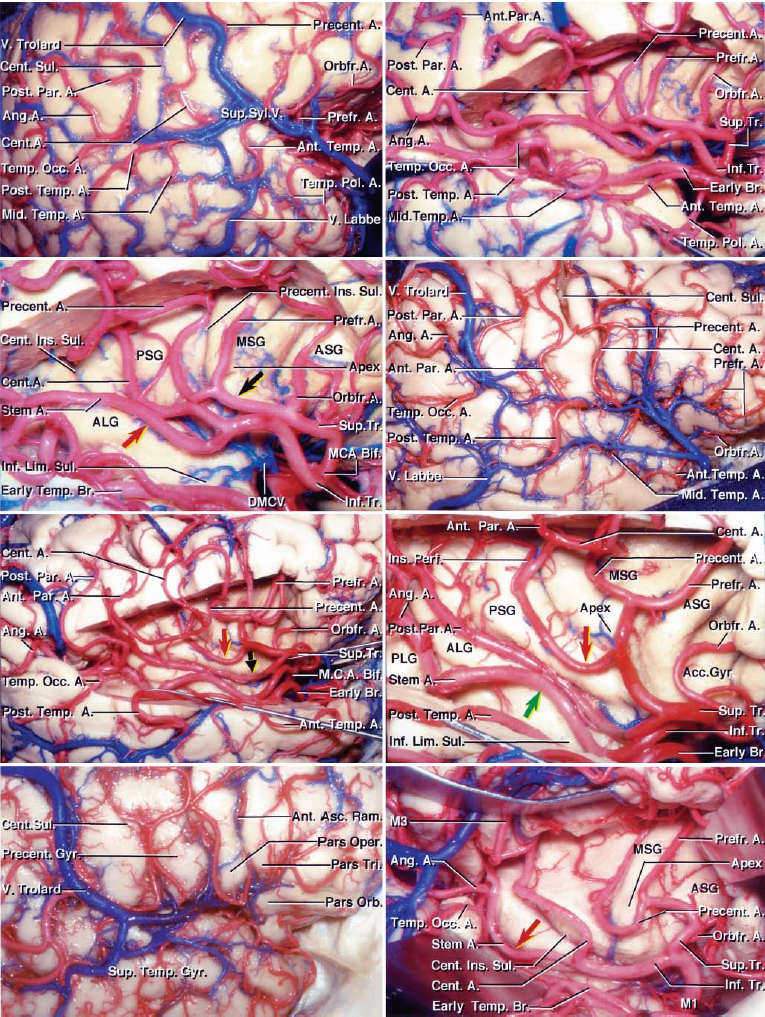
The arterial supply of the temporal lobe is from both the anterior circulation, via the inferior trunk of the middle cerebral artery (MCA) branches coursing through the Sylvian fissure, and posterior circulation, via the posterior cerebral artery (PCA) branches coursing through the crural and ambient cisterns.
As a general principle, the Sylvian and lateral aspects of the temporal lobe are fed mostly by the MCA branches; the inferior or basal aspects are supplied mostly by the PCA branches. Anteromedial temporal lobe structures, including the amygdala and hippocampus, are supplied by the anterior choroidal artery, the anterior thalamoperforatoring branches of the posterior communicating artery (PCoA) and the PCA perforating arteries. Posteromedial parts of the lobe are dependent on the PCA branches.
These MCA and PCA systems disproportionately supply temporal arteriovenous malformations (AVMs) based on the location of the nidus; their contributions to each AVM should be studied preoperatively.
The proximal MCA trunk (M1) has two early branches supplying the temporal pole:
- Temporopolar artery, distal to ICA bifurcation
- Anterior temporal artery, proximal to the MCA bifurcation
These two branches originate from the inferior surface of the M1 trunk and feed most of the anteromedial temporal AVMs.
The M1 terminates in three different patterns: 1) bifurcation into the superior (frontal) and inferior (temporal) trunks, 2) trifurcation into the superior, middle, and inferior trunks, or 3) division into multiple trunks.
The inferior trunk courses through the Sylvian cistern and composes M2 (insular) and M3 (opercular) segments. These segments later emerge from the Sylvian fissure to create the M4 cortical branches, including (from anterior to posterior):
- Middle temporal artery
- Posterior temporal artery
- Temporo-occipital artery
- Angular artery
These branches are collectively referred to as superior temporal arteries.
Click here to view the interactive module and related content for this image.
Figure 1: The branches from the inferior MCA trunk provide most of the feeding vessels to the Sylvian and lateral temporal AVMs (images courtesy of AL Rhoton, Jr).
The posterior temporal artery arises from the PCA and provides branches to the medial and the basal temporal lobe via:
- Hippocampal arteries originate within the crural and ambient cisterns and supply the uncus, anterior parahippocampal gyrus, hippocampus, and the dentate gyrus.
- Posterior temporal artery, including its anterior, middle, and posterior branches, originate as individual branches or all from a common trunk and supply the basal temporal lobe.
As a whole, these branches are called inferior temporal arteries. These branches may pass around the basal surface and supply the middle temporal gyrus.
Click here to view the interactive module and related content for this image.
Figure 2: Microsurgical anatomy of the medial and basal temporal lobe as related to AVM surgery. The en passage vessels should be differentiated from the AVM’s terminal feeders. The naming of P2 branches has been inconsistent among different authors (images courtesy of AL Rhoton, Jr).
Venous drainage is quite variable and is routed to different superficial and deep venous systems according to the location of the AVM. The AVMs on the lateral surface typically drain into the descending veins, joining the vein of Labbe and ultimately the transverse sinus. The AVMs on the basal and the medial surfaces drain into the tentorial sinuses, rarely into the sphenobasal and sphenopetrosal and/or medially into the basal vein of Rosenthal. The superficial temporal veins drain the superficial Sylvian AVMs.
TEMPORAL AVM SUBTYPES
Lateral Temporal AVM
The dominancy of the feeders for this subtype differs according to the location, size, and complexity of the nidus. These feeders mainly include the anterior temporal and temporopolararteries at the anterior border; branches of the inferior trunk of the MCA at the superior border, as well as posterior temporal and temporo-occipital arteries at the posterior and inferior margins of the AVM.
Figure 3: Lateral temporal AVMs are cone-shaped lesions based on the lateral temporal convexity, often extending deep to the level of the temporal horn ependymal surface. Because the lateral aspect of the lobe is its largest surface, this AVM subtype is the most common subtype among the temporal AVMs. Venous drainage is also variable depending on the location and complexity of the nidus, but it is mostly superficial and descends into the vein of Labbé. The drainage can also ascend toward the superficial Sylvian vein and sphenoparietal sinus. In large lesions extending to the ependymal surface of the temporal horn, there are small feeders from the anterior choroidal artery and the P2 segment at the deeper part of the parenchymal dissection.
Figure 4: A simple lateral temporal lobe AVM is demonstrated. For more inferiorly located temporal lesions on the inferior temporal gyrus, the posterior temporal arteries from the P2 segment is also involved at the posterior border of the nidus. Note the large draining vein coursing posteriorly (lower row-lateral and anteroposterior ICA angiograms). The predominance of the superior temporal artery feeders is apparent.
Lateral Temporal AVM: Commando Operation
For posteriorly located lesions on the dominant hemisphere, an important consideration is preservation of the Wernicke’s speech area, located about 5 cm from the temporal pole along the middle and superior temporal gyri. I do not advise preresection mapping or the use of an awake craniotomy for resection of any AVM. Compact nidi may be removed with a low risk of permanent speech dysfunction through the transsulcal approach.
The craniotomy is planned according to the location of the lesion. For anteriorly located lateral temporal AVMs (in front of the external auditory canal), a tailored pterional craniotomy is reasonable. A temporal craniotomy is more appropriate for posterior temporal AVMs. Both of these approaches are performed with the patient in the supine position with contralateral head rotation. The head rotation is 90 degrees for the temporal craniotomy to make the midline axis positioned horizontally.
The extent of the craniotomy and dural opening should be generous. Small transdural feeders from the middle meningeal artery may supply the AVM, and the operator should look for these to prevent their avulsion during dural mobilization. Dural opening should accommodate the safety of the vein of Labbe.
Arachnoid dissection starts through the sulcal planes and follows the large feeding vessels in a circumferential manner; the arterial feeders and draining veins are clearly identified. The feeding arteries should be pursued to the level of the nidus and only then sacrificed because some of the cortical Sylvian feeders may be en passage and on their way to supply the language cortices or other unaffected areas of the brain.
The deep feeders should also be interrupted in a circumferential manner by parenchymal dissection as close to the nidus as possible. Intrasulcal dissection of small compact AVMs within the Wernicke’s area is safely possible.
Large AVMs extend into the temporal horn and receive feeders from the anterior choroidal arteries; this artery’s main trunk and nonfeeding perforators should be carefully preserved proximally within the choroidal fissure. The dominant hippocampus should be spared to avoid disabling memory dysfunction.
Disconnection of the high-flow P2 segments leading to large AVMs may cause retrograde thrombosis of the PCA and partial visual field defects.
All venous drainage to the vein of Labbé or superficial temporal veins should be saved intact until the completion of thorough circumdissection.
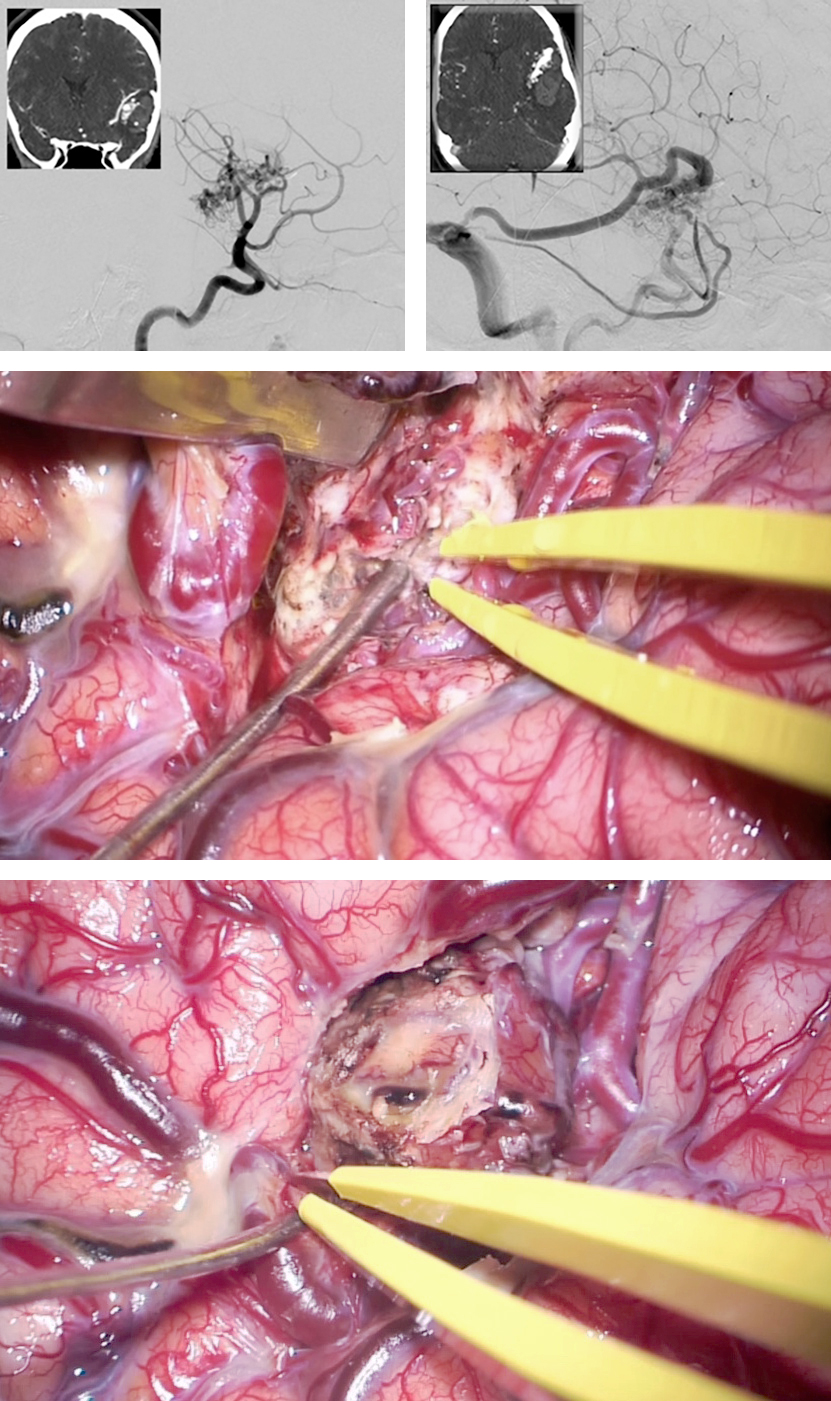
Figure 5: A large complex right-sided lateral temporoparietal AVM is shown (lateral and anteroposterior ICA injections-upper images). The MCA feeders (left lower image) and P2 feeders were clipped and transected. Temporary venous occlusion (right lower photo) confirmed adequate nidal devascularization, after which the AVM was delivered.
Giant AVMs: Techniques for Resection
Figure 6: A ruptured lateral temporal AVM underwent resection. Note the large arterialized vein of Labbe (first row). A right temporal craniotomy was completed using a linear incision and the large distal MCA feeding vessel was identified by means of intersulcal dissection(secondrow). This vessel was assumed to be en passage and provided numerous feeders to the AVM along its length. The nidus was situated just superior to this en passage artery;the artery was skeletonized and the nidus removed (last row). Note the darker vein of Labbe at the end of resection. The preservation of en passage arteries is important for avoidance of distal ischemia.
Basal Temporal AVM
Basal temporal AVMs sit within the inferior temporal gyrus, fusiform, and/or parahippocampal gyri. The nidus may not have direct cortical presentation unless generous subtemporal dissection and tangential viewing is attempted. This tangential working angle makes resection of this type of AVM more challenging than lateral temporal AVMs.
Figure 7: Feeders are mostly arising from the inferior temporal arteries, originating from the P2 segment of the PCA. These PCA feeders are encountered at the medial border of the AVM after anterior and posterior circumferential dissection. Superior temporal arteries may be recruited at the lateral margin. Venous drainage is typically superficial through temporobasal veins en route to the vein of Labbé and potentially to the basal veins of Rosenthal.
Basal temporal AVMs are approached via temporal/subtemporal craniotomy with the patient in the supine or lateral position; the midline axis of the head is positioned horizontally. I tilt the head toward the floor to exploit the help of gravity, mobilizing the temporal lobe away from the middle fossa floor. I routinely use the lumbar drain to achieve early temporal lobe relaxation.
The inferior border of the craniotomy should be drilled away so that it is flush with the level of the middle fossa floor. Mastoid air cells are frequently entered and are packed with bone wax. The dural opening is based inferiorly. The vein of Labbé is protected during the posterior dural incision because this vein may be interdigitated within the dural leaflets before joining the transverse sinus. If this is the case, I open the dura around the vein and leave a piece of the dura intact on the vein to preserve its integrity.
Lumbar CSF drainage is used (10cc aliquots, maximum 80cc) to obviate the need for aggressive lobar retraction. Subtemporal dissection should be performed cautiously so that small meningeal feeders from the middle fossa dura are not inadvertently avulsed.
The main obstacle for temporal lobe mobilization is the vein of Labbé that tethers the lobe to the floor. This is especially problematic for posteriorly located lesions where the surgeon should avoid premature avulsion of the vein. After exposure, the subdural segment of the vein may be first untethered from its surrounding arachnoid layers, and then covered with wet cottonoid patties to prevent its desiccation and rupture.
Sulcal dissection starts at the lateral margin of the AVM, interrupting the feeders from the superior temporal arteries, and progresses circumferentially and deeply, but perpendicular/oblique, to the axis of the nidus. Inferior temporal feeders are exposed after lateral, anterior, and posterior dissection and mobilization of the nidus into the subtemporal space. In this way, the posterior temporal arteries are exposed and interrupted along the medial margin at the tentorial incisura. The apex of the malformation, fed by choroidal arteries, can invade the wall of the temporal horn and be a source of significant bleeding; this area is within the blind spot of the operator.
The limited working space within the medial edge of the lesion can complicate the control of bleeding. Therefore, selective embolization of the inaccessible PCA feeders is a reasonable strategy.
Sylvian Temporal AVM
This AVM subtype is situated on the Sylvian surface of the superior temporal gyrus and fed primarily by the MCA inferior trunk at its superior and medial aspects. Depending on the reach of the nidus into the temporal horn, the anterior choroidal artery and its branches may also participate in the lesion.
Figure 8: The feeding and draining vessels as well as the relationship of the surrounding structures for this AVM subtype is illustrated. Note the reach of the AVM nidus into the temporal lobe. The superficial and deep Sylvian draining veins irrigate this lesion.
Sylvian temporal AVMs do not invade the pial surface of the frontal opercula and insular cortices. They may therefore have no cortical presentation on the lateral aspect of the superior temporal gyrus except for an arterialized draining vein that is apparent as a landmark. Venous drainage is superficial and involves superior and deep Sylvian veins.
Figure 9: A ruptured left-sided Sylvian temporal AVM is demonstrated. The early and late arterial phases of the oblique ICA angiogram are included (upper images). Wide Sylvian fissure dissection allowed skeletonization of the MCA branches and disconnection of the AVM feeding vessels (middle photo). Following disconnection of the nidus, the draining veins were sacrificed; the remaining cortical veins turned dark blue (lower photo).
The patient’s position and craniotomy are the same as those for frontal Sylvian AVMs. The supine position is used via a pterional craniotomy. I advocate a generous and deep Sylvian fissure split while leaving arterialized superficial Sylvian veins on the temporal side of the fissure and skeletonizing the M2 and M3 trunks. These maneuvers allow me to differentiate terminal feeders, transit arteries, and bystanders within a fissure that is full of various vital branches.
Sulcal and pial dissection starts at the superamedial side of the lesion to interrupt the feeders, leaving the transit arteries and bystanders intact during proximal to distal dissection of the MCA feeders. Circumdissection isolates the apex of the nidus within the temporal horn where the choroidal vessels are found and divided.
Sylvian temporal AVMs may be located posteriorly and involve the Heschl’s and Wernicke’s gyri. Meticulous microsurgery near the AVM’s compact nidus allows removal of these AVMs with minimal permanent risks.
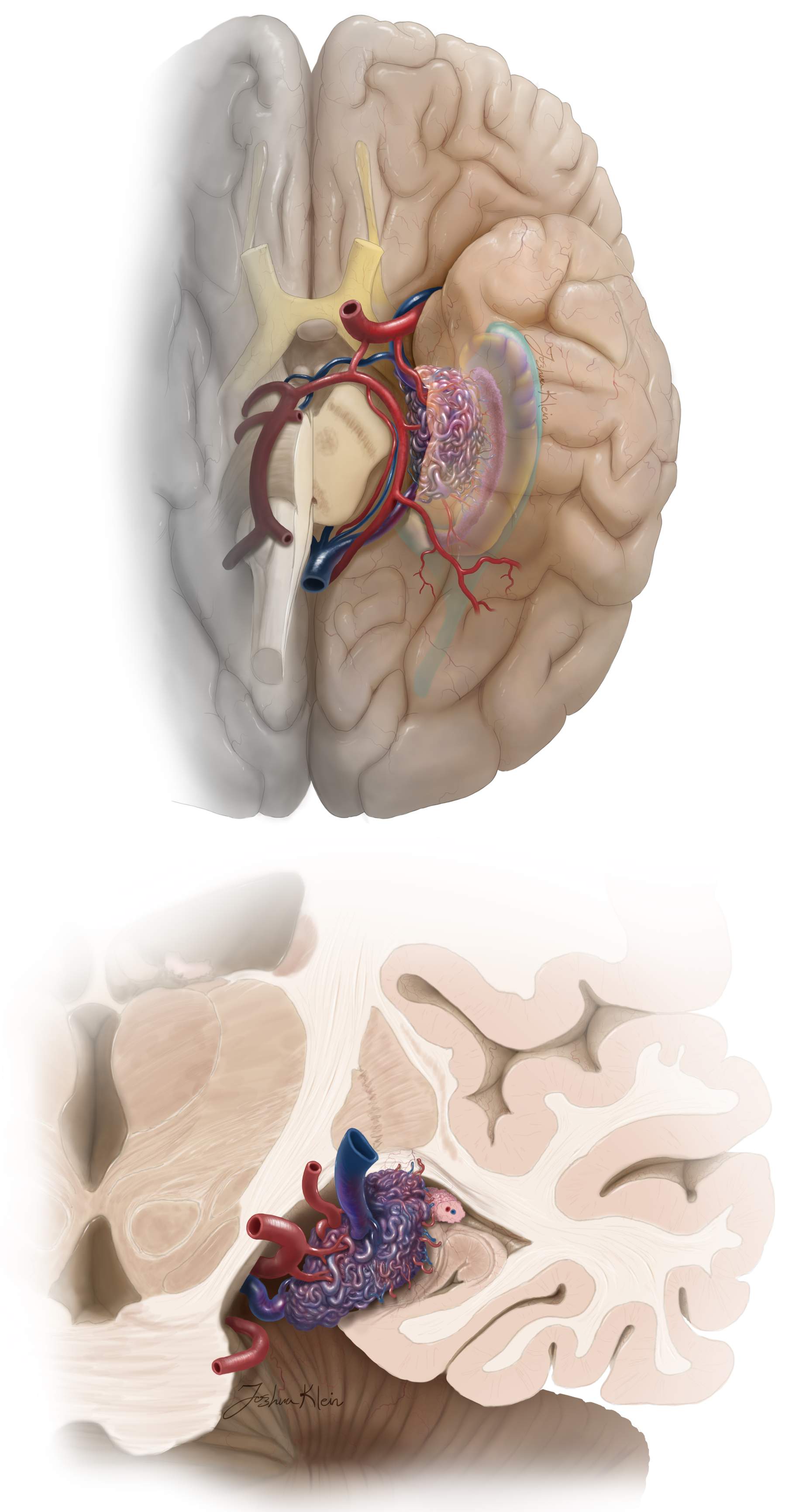
Medial Temporal AVM
Medial temporal AVMs primarily involve the uncus, hippocampus, and parahippocampal gyrus without any cortical representation. Based on their size, these lesions can infiltrate the basal ganglia.
Feeders originate from the anterior choroidal artery, anterior temporal artery, thalamoperforator branches of the PCoA, hippocampal artery, posterior temporal arteries, and the P2 segment of the PCA. Venous drainage is routed medially toward the basal vein of Rosenthal and occasionally anterolaterally toward the superficial and deep Sylvian veins.
Exposure of these AVMs is tailored based on the anteroposterior location of the nidus in relation to the hippocampus.
Anteromedial Temporal Lesions
For lesions anterior to the mid portion of the hippocampus, I prefer an orbitozygomatic craniotomy or more frequently, an extended pterional craniotomy. These approaches offer a short working distance and wide working space through the transsylvian corridor. The working angle is tangential.
Figure 10: A left-sided anteromedial temporal AVM is shown on an oblique ICA angiogram (top image). CT angiogram images (insets) localized the AVM. The feeding vessels were arising from the anterior temporal artery, thalamoperforatoring branches of the PCoA, hippocampal artery, posterior temporal arteries, and the P2 segment. Venous drainage was routed medially toward the basal vein of Rosenthal. I resected the lesion via the transsylvian approach. The anterior temporal artery was draped over the AVM (lower image).
Figure 11: Generous Sylvian fissure splitting and opening of the carotid and basal cisterns allows skeletonization of the anterior choroidal artery, P2 segment, and occlusion of the terminal feeders to the medial aspect of the AVM. En passage midbrain perforators are left intact. Note the draining vein coursing posteriorly over the uncus. The MCA (under the suction device) is exposed.
Figure 12: The feeding vessels from the anterior temporal artery (left) and the M1 trunk (right) are isolated and sacrificed.
Figure 13: The other feeding vessels from the P2, PCoA and anterior choroidal arteries are also disconnected.
The surgeon’s working angle is oblique to the axis of the AVM. The posteroinferior margin of the lesion is the least accessible border after the nidus is mobilized into the surgical corridor. The oculomotor nerve is carefully protected from the heat of the coagulation. Subpial resection of the uncus can protect the brainstem and its perforating vessels. Indiscriminate use of cautery in the event of excessive bleeding is not rewarding.
Figure 14: Ultimately, the AVM is completely disconnected, its draining veins transected and the nidus delivered.
Medial Temporal AVM
Posteromedial Temporal Lesions
For lesions posterior to the mid portion of the hippocampus, a temporal craniotomy, similar to the one for basal temporal AVMs, is appropriate. A transsulcal tranventricular exposure through the middle/inferior, fusiform or rarely the parahippocampal gyri is necessary.
Figure 15: The location and angioarchitecture of this AVM class is demonstrated. Note the feeding anterior choroidal artery and PCA tributaries within the choroidal fissure and ambient cisterns. The draining veins are typically deep and encountered at the end of the operation. The choroid plexus offers many feeding branches.
In this approach, my angle of view is also oblique to the AVM’s axis. Feeding vessels and draining veins are deep and encountered late in the dissection process. Anterior choroidal artery and PCA tributaries are found within the choroidal fissure and ambient cisterns, respectively. Their feeding vessels are found early and transected at the nidus. Dominant posterior hippocampal and parahippocampal involvement may be associated with postoperative memory impairment.
I have approached small and moderate sized posterior hippocampal and parahippocampal AVMs through the supracerebellar transtentorial approach with great success. This route provides a suitable operative corridor without any significant temporal lobe retraction or transgression. The anterior extent of exposure is limited, and therefore the appropriate AVM should be carefully selected. Please see the Transtentorial Approach to Parahippocampal Lesions chapter for indications and techniques.
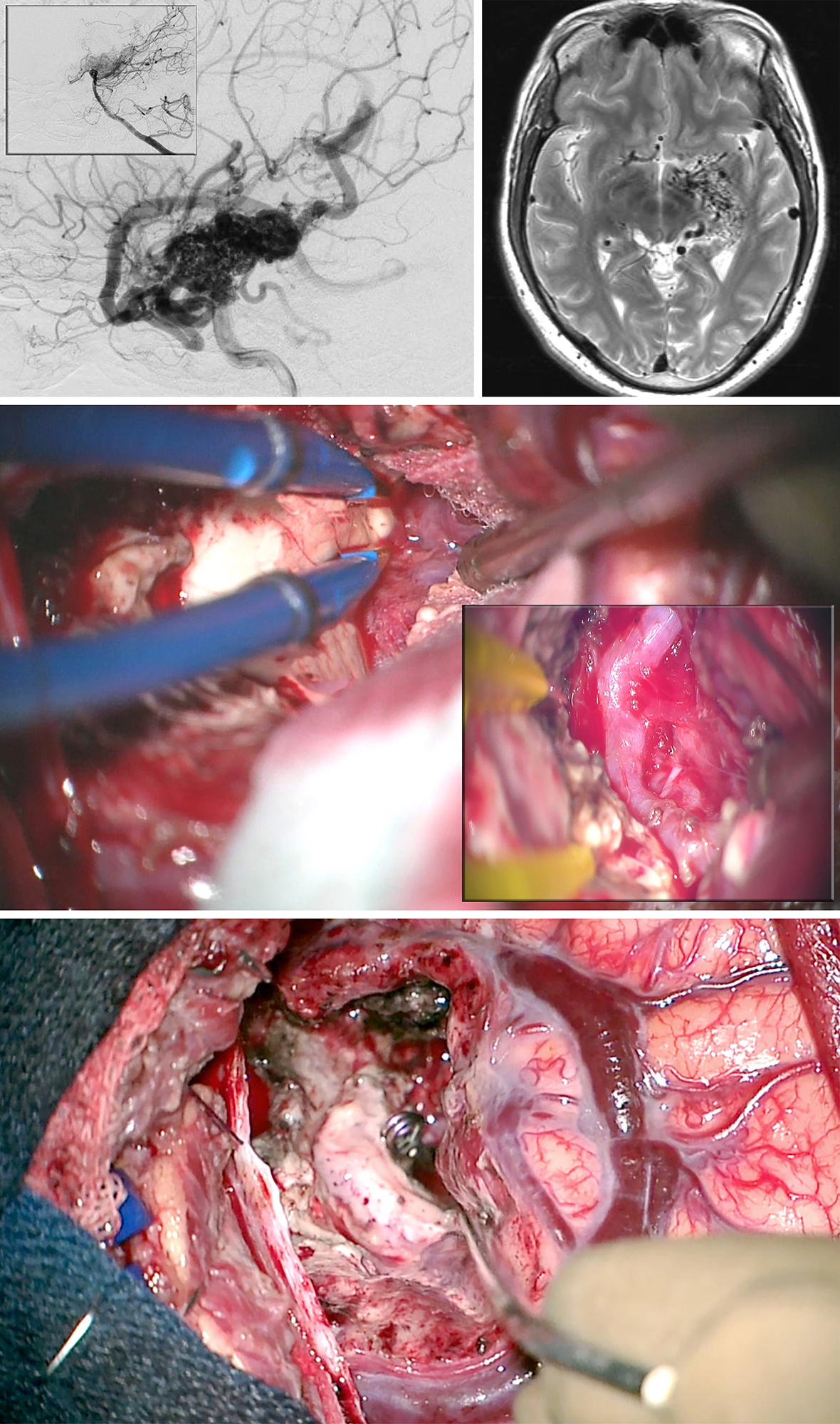
Figure 16: A large posterior medial temporal AVM, primarily fed by the anterior choroidal artery, is shown (lateral ICA and vertebral artery, inset, angiograms-upper images). I used the transcortical transventricular transchoroidal approach in this case (middle photo). The anterior choroidal artery was exposed within the choroidal fissure through the temporal horn (middle photo-inset). After disconnection of the AVM, the anterior choroidal artery was sacrificed at the level of the nidus and the AVM extracted (lower photo). All superficial veins turned dark blue.
Large Medial Temporal/Choroidal AVM
Figure 17: Another posterior medial temporal AVM is shown. Most feeders arose from the P2 branches (upper images). The hematoma cavity provided ample space for microsurgery via the transcortical approach. The hypertrophied P2 perforating branches were transected (middle images) and the AVM removed (lower photo).
Contributors: Rouzbeh Shams-Amiri, MD and Mohsen Noori, MD
References
Lawton, MT. Seven AVMs: Tenets and Techniques for Resection. New York, Stuttgart: Thieme Medical Publishers, 2014.
Please login to post a comment.