Parafalcine Meningioma
Figure 1: Cushing and a colleague (Dr. Gaston Decoppet) discuss the case of a patient with recurrent brain tumor.
This is a preview. Check to see if you have access to the full video. Check access
Large Parafalcine Meningioma: Principles of Resection
Parafalcine menigiomas originate from the falx, but unlike parasagittal meningiomas, they are completely covered by the cortex. They may grow through the falx and are often bilateral. Like their parasagittal counterparts, they are most commonly found around the middle third of the superior sagittal sinus, between the coronal and lambdoid sutures. They comprise 8.5% of intracranial meningiomas.
Diagnosis
Similar to their parasagittal counterparts, the clinical presentation of a parafalcine meningioma depends on its location along the falx. Tumors near the central sulcus may cause sensory and motor seizures or contralateral hemiparesis/hemianesthesia. Speech dysfunction may also occur if the tumor is related to the dominant supplementary motor area.
Accordingly, the tumors along the anterior third of the falx can reach a large size and may cause cognitive dysfunction, headaches, and blurred vision/papilledema. Those at the posterior third may cause visual disturbances such as homonymous hemianopsia or hallucinations. Occasionally, a falcine meningioma may be the source of intracerebral, subdural, or subarachnoid hemorrhage.
There is also the rare circumstance when the tumor can only cause isolated contralateral lower extremity weakness. This presentation should not be confused with radiculopathy because of nerve root compression. Upper tract signs (can be subtle) and distribution of the weakness (not respecting muscle groups for individual roots) help the clinician differentiate between these scenarios.
Evaluation
Magnetic resonance imaging (MRI) reveals an extra-axial interhemispheric tumor that has a dural tail and is based on the falx. T2-weighted MR images estimate the location of the distal anterior cerebral arteries and their branches. Parenchymal edema signifies pial invasion and correlates with neurologic deterioration after surgery.
Magnetic resonance or computed tomography venogram assesses the degree of superior sagittal sinus invasion or occlusion and estimates the location of large midline parasagittal bridging veins or their collaterals. The locations of these veins guide dissection, ensuring an adequate interhemispheric corridor to reach the tumor.
Large tumors often encase the callosomarginal and pericallosal arteries. A preoperative CT angiogram identifies displacement or encasement of these vital vessels.
Figure 2: Parafalcine meningiomas are located on the falx and can occur in a range of sizes (top images). A CT angiogram demonstrates encasement of the pericallosal and callosomarginal arteries by a very large tumor.
Other tumors that may mimic parafalcine meningiomas include metastatic tumors, osteochondromas and chondrosarcomas.
Indications for Surgery
Patients with progressive symptoms are reasonable candidates for surgery. Small asymptomatic tumors can undergo surveillance imaging. Tumors that partially obstruct critical bridging veins or the sinus may be subtotally removed and the growing tumor remnants treated with radiosurgery.
Preoperative Considerations
A lumbar drain affords brain relaxation that minimizes brain retraction (especially in the case of large tumors). This is especially important because cerebrospinal fluid (CSF) cavities are not entered during interhemispheric dissection to access the parafalcine tumor.
Elective cortical resection should not be necessary. I have not encountered any risk of transtentorial herniation with prudent intraoperative lumbar drainage for giant meningiomas causing significant mass effect and midline shift. CSF drainage is instituted upon opening of the dura and 10cc aliquots are gradually removed until adequate relaxation is achieved.
I do not advocate endovascular tumor embolization before surgery since the tumor can be readily devascularized from the falx early during dissection.
Advances in radiosurgery have affected my philosophy toward meningioma surgery. The goals of surgery, centered on patient safety, have evolved into favoring radical subtotal resection over aggressive total resection of tumors involving important arteries or veins. Radiosurgery is reserved for treating the growth of tumor remnants seen on follow-up surveillance imaging.
Falcotentorial meningiomas displace or encase the diencephalic (veins of Galen and Rosenthal) and straight sinus. Since the vasculature in this region is critical, a preoperative digital subtraction angiogram is beneficial to study these structures and their location in detail. Radical subtotal tumor resection ensures unharmed cerebrovascular architectures.
RESECTION OF PARASAGITTAL MENINGIOMAS
Please refer to the Interhemispheric Craniotomy or Parietal Craniotomy chapters for more details regarding accessing parafalcine meningiomas. There are certain considerations in craniotomy planning that are specific to parafalcine meningiomas.
Unlike parasagittal meningiomas, most parafalcine meningiomas can be readily resected through a linear incision. The incision is designed to cross the midline so that the superior sagittal sinus can be exposed by the craniotomy and mobilized through retraction sutures within the superior falx after dural opening. The lateral head position allows gravity retraction to mobilize the brain during operative maneuvers within the interhemispheric corridor.
Giant bilateral parafalcine tumors may be excised through a unilateral linear incision and transfalcine approach. The transfalcine corridor allows resection of the contralateral tumor without a need for a contralateral craniotomy and placing the contralateral parasagittal veins at risk.
Figure 3: A giant parafalcine tumor (top images) was approached through a unilateral linear incision and transfalcine route using gravity retraction. The lateral position mobilizes the dependent nondominant hemisphere away from the midline (middle image). The location of the tumor is illustrated through the overlay model (bottom image).
Giant Parafalcine/Falcotentorial Meningioma
The location of parasagittal veins has to be carefully studied on preoperative imaging. If the overlying ipsilateral parasagittal veins are numerous and obstructive, I have employed the contralateral transfalcine route to expose the tumor.
INTRADURAL PROCEDURE
A thin layer of normal cortex is usually covering the parafalcine tumor. Due to increased cranial tension, the vibrations of the drill during craniotomy can injure this thin cortex unless CSF drainage is used to achieve decompression. In addition, uncontrolled tension can lead to cerebral herniation; this can be especially problematic and consequential during exposure of the sensorimotor cortex.
Figure 4: To reach the tumor through the interhemispheric space, I incise the dura in a curvilinear fashion starting at its lateral edge, and create a dural flap based on the venous sinus. Care is taken not to injure any large draining veins. Occasionally, a small draining vein may need to be sacrificed. If a parasagittal vein is encountered draining into the sinus, the dural opening must be adjusted to protect the vein’s inlet into the sinus.
Figure 5: Large tumors may invade the convexity dura that can be resected during the dural opening while protecting the adjacent parasagittal veins. The tumor is first devascularized, then debulked and finally dissected from the surrounding cerebrovascular structures.
Wide dissection of the interhemispheric arachnoid membranes and untethering of the veins allow identification of the anterior and posterior poles of the tumor along the falx and localization of the deep neighboring vascular structures early in surgery. Lumbar CSF drainage is necessary for safe execution of this maneuver.
The tumor is first devascularized along the falx (left image). Again, lumbar CSF drainage promotes brain and tumor mobilization without a need for early tumor debulking, therefore exposing the tumor-feeding vessels arising from the falx. This maneuver allows aggressive tumor devascularization along most of the tumor base.
The mass is then enucleated by sharply cutting away tumor from the inside out (right image). Alternatively, an ultrasonic aspirator may be used. Next, the tumor capsule can be pulled into the enucleated cavity while maintaining the extrapial dissection planes.
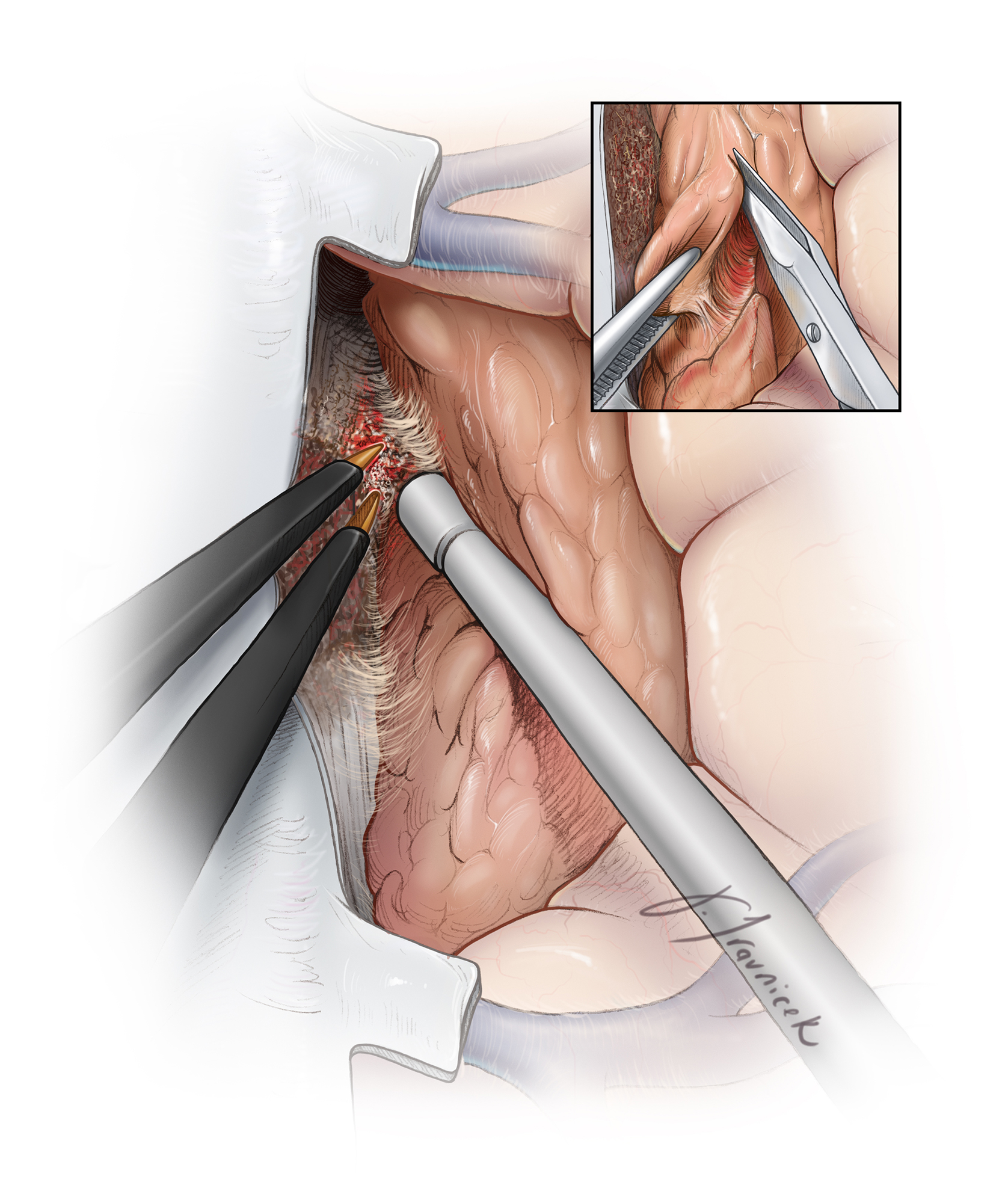
Figure 6: The tumor capsule is mobilized away from the brain along the extrapial dissection planes, and the capsule is progressively shrunken using bipolar electrocautery. I use cottonoid patties to circumferentially cover the brain and develop and maintain a plane between the tumor capsule and the pia mater. Notice that the plane is developed along the circumference of the tumor evenly instead of superficially to deep in a single spot. This allows easier mobilization of the mass (inset image) and avoids small working areas in case of bleeding.
If the integrity of the pia is violated, the cottonoid patties are used to “wipe” the brain away from the tumor while the enucleated tumor capsule is drawn into the resection cavity (inset image). The patties are ‘rolled’ into place toward the deepest aspects of the dissection cavity to create the plane. The critical vessels (the callosomarginal and pericallosal arteries) may be adherent to the tumor capsule and must be microsurgically and sharply dissected away. Each artery should be carefully inspected to determine its function as an en passage versus a tumor-feeding vessel.
It may be necessary to leave a small piece of tumor over the en passage vessels to protect their integrity. Aggressive arterial manipulation should be kept to a minimum to avoid arterial spasm.
Premature traction on the inferior pole of the tumor without adequate inspection can lead to vascular avulsion injury and disappointing results. Finally, the resection cavity and dural edges are inspected for residual tumor.
Figure 7: After removal of the tumor, the section of the falx invaded by the tumor may be excised using a curved knife to cut the falx toward the superior sagittal sinus. The inferior sagittal sinus may be sacrificed if the superior sagittal sinus is patent.
Extension of the tumor to the contralateral side of the falx requires transection of the falx and creation of an operative window to reach the tumor. Alternatively, the craniotomy may be extended to the other side of the sinus and the tumor exposed through a contralateral paramedian dural opening. I prefer the ipsilateral interhemispheric transfalcine approach since it does not place the contralateral parasagittal veins at risk.
Case Example
The following case demonstrates the intraoperative events related to resection of a large bilateral parafalcine meningioma.
Figure 8: This bilateral parafalcine meningioma (upper row) was removed via a unilateral right-sided parasagittal transfalcine approach. Traction sutures in the superior falx (second row) gently mobilized the superior sagittal sinus. The tumor on the right-side of the falx was aggressively devascularized, debulked and the callosomarginal artery dissected (third row). Ultimately, further tumor removal uncovered the pericallosal artery and the contralateral tumor was excised via the transfalcine approach and removal of the affected section of the falx (arrows)(last row).
BiIateral Parafalcine Meningioma: Managing Vascular Adherence and Maximizing Resection
Bilateral Parafalcine Meningioma
Posterior Parafalcine Meningioma
Closure
The previously harvested pericranial graft may be sutured in place if a portion of the dura was resected. Tack-up stiches are used to prevent formation of epidural hematoma and fluid collections.
Postoperative Considerations
A postoperative MRI is obtained. Steroids may be slowly weaned as tolerated by the patient. Prophylactic anticonvulsants are administered perioperatively, but tapered off 1 week after surgery if the patient has not suffered from a seizure. The patient is kept well-hydrated during the immediate postoperative period to decrease the risk of venous thrombosis, especially if the venous sinus or parasagittal veins were manipulated or sacrificed during the operation.
Pearls and Pitfalls
- Preoperative studies must be reviewed (including T2-weighted MR images) to approximate the location of the distal anterior cerebral arteries.
- Sacrifice of the bridging veins should be kept to a minimum.
- The trigeminocardiac reflex may cause bradycardia or asystole when the falx is manipulated.
Please login to post a comment.