Anterior Circulation Aneurysms: Clip or Coil?
PERSONAL PHILOSOPHY TO ANEURYSM CARE
Treatment of intracranial aneurysms has evolved over the past decade through the advent of innovative endovascular and refined microsurgical techniques. Most importantly, therapy should be individualized because each patient has a unique set of medical and pathoanatomic features that factor into the decision-making process for treatment. This phenomenon is especially true for aneurysm care.
Each aneurysm must be assessed for its size, shape, location, history of rupture, and presence of calcification or thrombus. The patient’s age, neurologic and clinical status, and family history are also important factors in estimating the natural history of each intracranial aneurysm. The optimal treatment strategy should provide benefit over the natural history of the aneurysm and aim for low rates of morbidity and high rates of aneurysm obliteration.
Outcome and complication rates of a treatment for a particular aneurysm are confounded by the surgeon’s training and recall biases. Existing clinical trials are not always conclusive because the success of treatment in the hands of a particular surgeon or interventionalist most likely reflects that surgeon’s or interventionalist’s experience and technical skill. Therefore, proper judgment and judicial interpretation of the literature are advised.
Microsurgical clipping has traditionally been considered the gold standard for treating intracranial aneurysms; however, advances in endovascular technology in the last two decades have shifted clinical practice away from microsurgical clipping in many institutions. Intracranial guide catheter access, balloon-, stent- and other device-assisted coiling, as well as flow diversion technology, have made endovascular treatment of nearly all intracranial aneurysms technically possible.
Therefore, technical feasibility is rarely an important consideration in the determination of endovascular aneurysm treatment as it was in years past. The effectiveness as well as the completeness and durability of treatment are the more appropriate considerations.
Equally comprehensive microsurgical and endovascular expertise should be available at each center of excellence so the only factor that determines treatment choice is the patients’ needs and not the expertise of the personnel.
Where is the “sweet spot” for the proportion of the anterior circulations aneurysms that should be coiled and clipped? Although the referral patterns can govern this proportion, I believe ~70% coil and 30% clip currently demonstrates a healthy approach to treatment.
This chapter focuses on the nuances of selection of surgical and endovascular modalities for anterior circulation aneurysms. Some controversy remains regarding the optimal management strategy for certain lesions.
However, other patients such as the ones suffering from life threatening hemorrhages with mass effect should undergo surgical decompression and clip ligation of their aneurysm. These “clear-cut” scenarios will not be reviewed here.
Recurrence and Rerupture
Microsurgical techniques result in low rates of postclipping recurrence/rupture and are therefore considered highly efficacious, but success is not absolute. David and colleagues followed patients with surgically clipped aneurysms angiographically with a mean follow-up of 4.4 years and found a 1.5% rate of aneurysm recurrence despite documented initial postoperative obliteration. In their series consisting of 102 patients harboring 160 aneurysms, one patient suffered from subarachnoid hemorrhage from a recurrent aneurysm despite complete microsurgical clipping.
The overall success rate of microsurgical clipping is approximately 98.5% across all aneurysm types and locations. A meta-analysis performed by Raaymakers et al. appraised 61 reported studies, including 2,460 surgically treated patients, and estimated the overall morbidity and mortality among these patients to be 10.9% and 2.6%, respectively. This study included complex and symptomatic aneurysms.
Several other studies estimated the mortality and morbidity of microsurgery for unruptured and asymptomatic intracranial aneurysms between 1% and 4.1%, respectively. Complications from surgery are more likely with aneurysm-associated mass effect, cerebral ischemia, subarachnoid hemorrhage, larger aneurysm size, complex morphology, and older patient age. The most important adverse effect is the neuropsychological decline related to microsurgery. Unfortunately, this specific effect has not been adequately studied but is a real and underestimated risk.
Endovascular techniques have established their safety superiority in comparison with clipping. There remains some limited controversy regarding their durability in select aneurysms.
The overall risk of recanalization after endovascular therapy has been reported as high as 49% for small aneurysms and up to 90% for giant or wide-neck variants, necessitating retreatment. Despite these shortcomings, an 11-year follow-up study found the overall rate of aneurysm rupture 1.6% after endovascular treatment—comparable to microsurgical clipping. In other words, although recanalization and the need for retreatment remain the primary limitations of endovascular therapy, the risk of SAH from the small recurrent and residual aneurysms is exceedingly small.
Technical Considerations for Endovascular Aneurysm Treatment
Although this Atlas is primarily focused on microsurgical techniques, some of the basic technical concepts of endovascular therapy will be reviewed herein.
When the decision to proceed with endovascular therapy is made, the surgeon should consider the achievable proximal catheter support given the patient’s vascular tortuosity and the need for stents or other intraluminal devices. The safety of dual antiplatelet therapy, particularly in patients with ruptured aneurysms, is an important consideration. The size of the parent vessel and the eloquence of adjacent branches/perforators are additional decisive factors.
Coil embolization and deployment of intraluminal assist devices (balloons, stents, and flow diverters) require navigability and near 1:1 transmission of axial forces that might be compromised in patients who own challenging proximal tortuosity. Although technology is improving rapidly, more complicated intraluminal maneuvers are needed for deploying certain embolization devices, particularly the newer braided stents and flow diverters. Larger, stronger, and more navigable guide catheters have made routine intracranial guide catheter access achievable. Insufficient proximal support may increase device complications and decrease the efficacy of endovascular options.
Balloon-assisted coil embolization can be effective for tackling many wide-necked aneurysms; stent-assisted coil embolization may have the additional benefit of flow alteration, either by creating some degree of flow diversion or by mechanically altering the angle of the aneurysm relative to the inflow jet from the parent vessel.
Flow diversion has also emerged as a viable option in broad-based, dysplastic, and fusiform aneurysms. Intraluminal devices require dual antiplatelet therapy to prevent thrombotic complications; therefore, careful consideration of the safety of dual antiplatelet therapy, particularly in patients with ruptured aneurysms and acute hydrocephalus requiring ventriculostomy or shunting is warranted.
The interventionalist must consider the size of the parent vessel to understand the ability of the vessel to accommodate the available intraluminal devices. The size of the aneurysm neck relative to the parent vessel may also reflect the ability of endovascular treatment to preserve the parent vessel lumen. Aneurysms arising from tiny or small vessels may be more amenable to microsurgical clipping or parent vessel sacrifice than to coiling.
Finally, the covering of eloquent branches or perforators with intraluminal devices can be performed even with flow-diverting stents, but the risk of damage to perforator-rich zones and eloquent branches must be included in the selection process for endovascular treatment. Another quandary for endovascular surgeons is the preservation of occasional vessels that directly arise from the aneurysm dome. Although balloon-assisted coiling, flow diversion, and advances in framing coil technology have provided tools for preserving vessels arising from the dome, microsurgical reconstruction of the vessel origin should be considered judiciously as an alternative.
COMPARISON OF INDIVIDUAL LOCATIONS
Although every aneurysm has characteristics that make it amenable to one treatment or another, there are certain factors associated with each aneurysm location that tend to favor either open microsurgical management or endovascular therapy.
The majority of anterior circulation aneurysms are saccular aneurysms arising at branching points of the major cerebral arteries. The treatment decision for anterior circulation aneurysms should consider the relationship of the aneurysm to the adjacent perforating vessels, and in the case of internal carotid artery (ICA) aneurysms, the relationship to the anterior clinoid process.
Paraclinoid and Ophthalmic Artery Aneurysms: The Case for Microsurgical Clip Ligation
Ophthalmic segment aneurysms include (from proximal to distal): medial carotid cave, ophthalmic, and superior hypophyseal aneurysms. Surgical access to this location requires skull base osteotomy techniques in addition to the standard transsylvian exposure. These supplementary adjuncts involve dissection of the clinoidal triangle. This anatomic triangle consists of the anterior clinoid process, its associated dural rings, and the cavernous sinus.
Ophthalmic artery and superior hypophyseal artery aneurysms encompass the majority of paraclinoid aneurysms. Other aneurysms at this location include carotid cave, dorsal wall, and ventral wall variants, but these types are relatively rare. Surgical exposure of this group of aneurysms requires removal of the anterior clinoid process, as well as reflection of the distal dural ring.
Ophthalmic aneurysms generally arise from the superior wall of the ICA and project cranially toward the optic nerve, this configuration allows for more standard surgical clipping with minimal dural ring resection. The optic nerve is very vulnerable to manipulation, and for this reason, the falciform ligament and optic canal must be unroofed to facilitate aneurysmal neck dissection.
On the other hand, superior hypophyseal aneurysms arise from the inferomedial wall and project medially. The inferomedial wall of the ICA is within the operative blind spot of the surgeon; therefore, angled fenestrated clips and thorough dural ring dissection are obligatory for safe clipping. Inadequate mobilization of the distal dural ring can impede clip deployment and prevent clip advancement toward the proximal aneurysm neck. The medial perforators supplying the optic apparatus must be carefully preserved before clip placement.
Dorsal wall aneurysms are often more straightforward to expose; however, they are often difficult to clip. These aneurysms arise from the superior wall of the ophthalmic segment of the ICA and are considered pseudoaneurysms as there is no relationship with the branching vessels emerging from the ICA. They are blister shaped, and may be a result of arterial dissection.
Their incompetent walls and small stature render these lesions technically challenging for both surgical and endovascular therapy. Minor manipulations of blister aneurysms can lead to their avulsion and catastrophic hemorrhage. Also, simple clipping is often inadequate to treat dorsal wall aneurysms as the pathologic aneurysmal dilatation extends into the wall of the ICA.
Therefore, ICA trapping with or without distal bypass may be necessary. Wrapping and the use of encircling clips are alternative adjuncts, but recurrences can nevertheless occur. The natural history of these lesions leads us to believe that this disorder is a segmental vascular disease rather than a focal problem, and combination therapy may be considered in these challenging cases.
Carotid cave aneurysms arise even more proximal on the medial surface of the ICA than the superior hypophyseal aneurysms and require additional mobilization of the ICA out of the carotid sulcus for reasonable operative visualization. Such extensive dissection and manipulation increases the risk of complications. These aneurysms are excellent candidates for endovascular and flow diversion therapies.
Postoperative blindness is a real risk of microsurgical intervention for paraclinoid aneurysms. Compromise of the vision can occur as a result of direct manipulation, injury to optic nerve perforators, or heat-related injury transmitted from drilling near the optic canal. Overall, flow diverters have revolutionized endovascular therapy for paraclinoid aneurysms and play a primary role in their treatment.
Lets discuss some case examples to review the above concepts.
Case Example 1
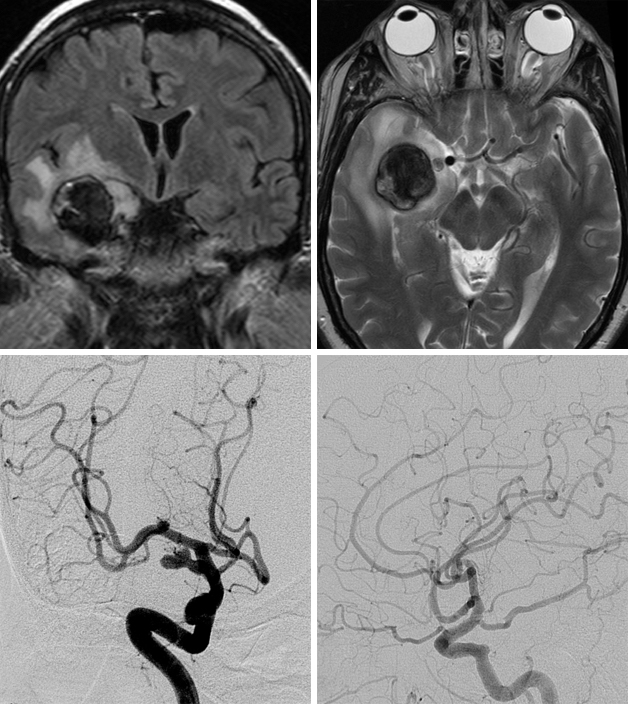
A 77 year-old female presented with headaches and personality change. MR imaging demonstrated a giant paraclinoid aneurysm with associated frontobasal edema. CTA showed an ophthalmic artery aneurysm. Given the presence of the mass effect and edema, microsurgical intervention was selected.
Figure 1: MRI demonstrates the thrombus within the aneurysm sac and surrounding mass effect and edema (top, left). CT angiogram reveals minimal blood flow within the sac (top, right). Postoperative CT scan shows partial resolution of the edema (bottom, left). Angiography confirms obliteration of the aneurysm via multiple clips (bottom, right).
Paraclinoid and Ophthalmic Artery Aneurysms: The case for Endovascular Intervention
Endovascular treatment of ophthalmic segment aneurysms is often the preferred modality because surgical intervention is complicated by inadequate exposure of the proximal neck, especially for more proximal aneurysms. On the other hand, complete coil embolization of ophthalmic, carotid cave, and superior hypophyseal aneurysms is also complicated by the proximity of this segment to the carotid siphon.
When the tortuosity of the ICA at the skull base prevents distal positioning of the guide catheter, the microcatheter may be pushed out of the aneurysm before satisfactory coiling achieved. Advancing a guide catheter into the cavernous segment, by using an angled microcatheter, or by using a balloon- or stent-assisted technique is a possible solution. In particular, a balloon may be used to hold the microcatheter in place within the aneurysm dome. Flow-diverting stents have been used in this location with great success, and elimination of the aneurysm appears more effective via flow diversion rather than by means of conventional endovascular treatment.
Case Example 2
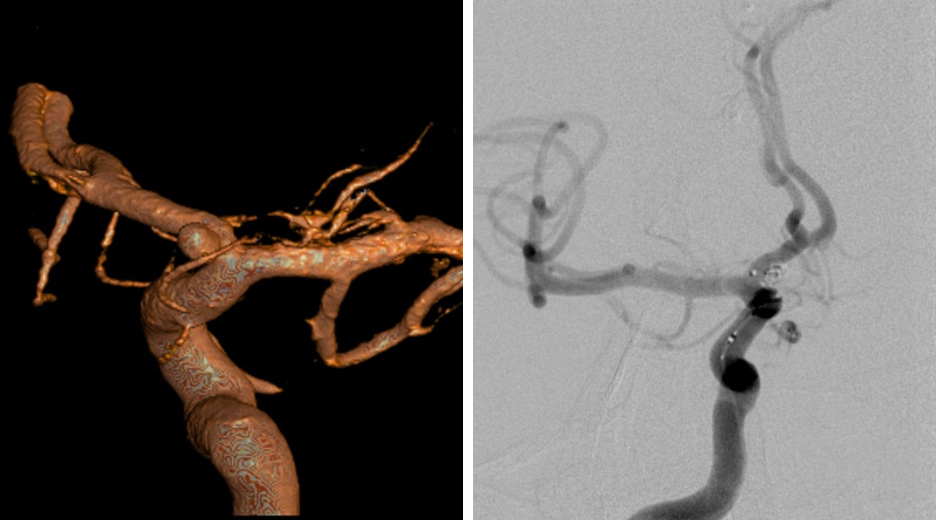
During diagnostic tests for headaches, a 62-year-old woman was found to have a left ophthalmic artery aneurysm. The vessel diameter was larger then the largest available pipeline device. Stent-assisted coiling of the aneurysm was performed and Raymond grade II embolization was achieved.
Figure 2: The left ICA demonstrates a superiorly projecting, bilobed aneurysm measuring 7mm (left image). Postoperative angiogram (right) demonstrates total occlusion of the aneurysm. Flow diversion has recently become the preferred intervention for paraclinoid aneurysms.
Posterior ICA Wall Aneurysms: The case for Microsurgical Clip Ligation
Posterior communicating artery (PCoA) aneurysms are often favorable for either surgical clipping or endovascular coiling. These aneurysms are frequently small and relatively easily accessible via both modalities. In the presence of oculomotor nerve palsy, microsurgery allows direct decompression of the oculomotor nerve and is more likely to lead to early recovery of this nerve’s function. In young (<50 years) patients, microsurgery remains the first treatment choice for PCoA aneurysms.
The domes of posterior and inferolaterally-projecting aneurysms have frequent adhesions to the oculomotor nerve, temporal lobe, or tentorium, increasing the risk of intraoperative rupture by operative manipulations during the early stages of the operation. The anterior clinoid process may also obscure the PCoA aneurysm base, necessitating partial removal of this bone. Lastly, large or wide-necked aneurysms may incorporate the PCoA and anterior choroidal arteries (AChoA), obviating the microsurgical approach and encouraging endovascular exploration via flow diverting devices.
The AChoA frequently originates from the neck of AChoA aneurysms, complicating endovascular attempts. As the AChoA is a small caliber vessel, it can be at a real risk during endosaccular embolization. Microsurgery continues to play an important role in treatment of this specific subtype of aneurysms.
Case Example 3
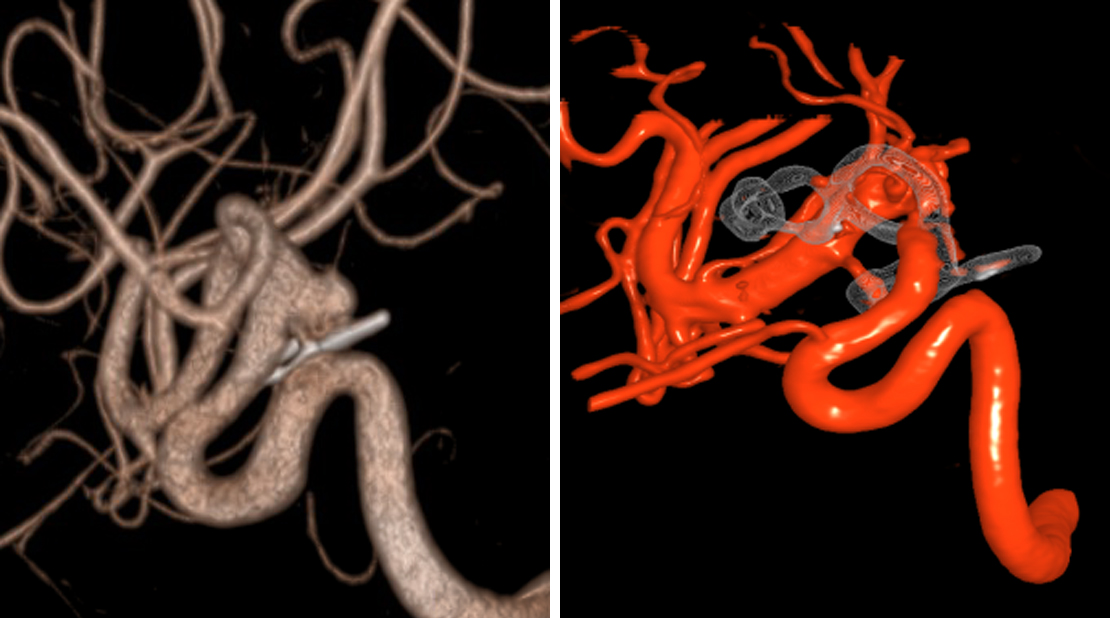
A 64-year-old man presented with seizures. The diagnostic work-up revealed a giant thrombosed aneurysm originating from the fetal-type PCoA incorporated at the base of the lesion. The aneurysm was successfully clip ligated after the thrombosed portion of the aneurysm was opened and evacuated.
Figure 3: FLAIR ant T2-weighted images (top row) reveal a partially thrombosed giant PCoA aneurysm with associated surrounding edema. Angiogram images of the right ICA show the living part of the aneurysm originating from the fetal type PCoA (left, bottom row). Postoperative angiogram demonstrates total obliteration of the aneurysm with preservation of the relevant cerebral vasculature (right, bottom row).
Case Example 4
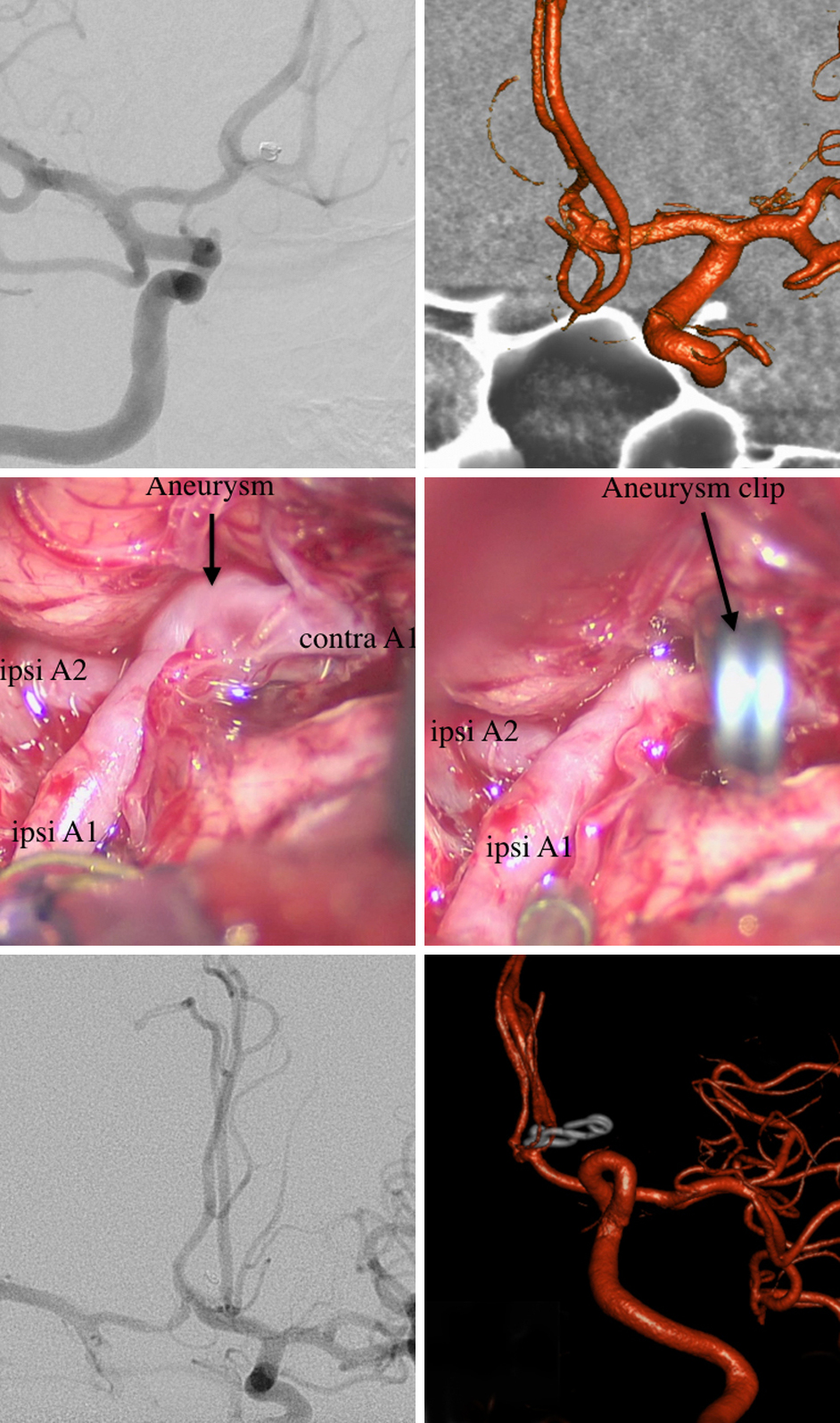
A 73-year-old woman suffered diffuse SAH from a ruptured left PCoA aneurysm. She underwent coil embolization. Follow-up formal cerebral angiogram 2 years later revealed regrowth of the aneurysm at the neck. She subsequently underwent clip ligation of her aneurysm.
Figure 4: Coil embolization of a ruptured PCoA aneurysm was accomplished (top row), however; aneurysmal regrowth at the neck was apparent 2 years later (bottom, left). Intraoperative findings for clip ligation of the recurrent aneurysm are shown (bottom, right). After application of the bayoneted clip, ICG angiography confirmed aneurysmal obliteration and PCoA patency.
Case Example 5
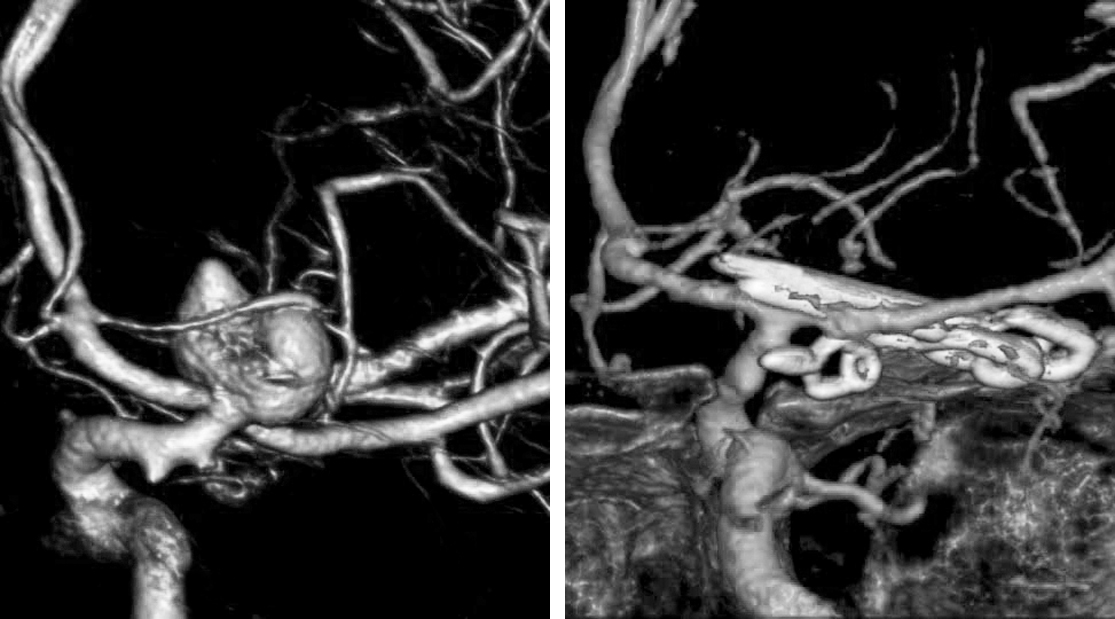
A 33-year-old woman with history of a prior SAH from a ruptured right PCoA aneurysm 5 years previously underwent clip ligation of her right-sided aneurysm at an outside institution. She later presented with regrowth/residual aneurysm and underwent repeat clipping.
Figure 5: Three-dimensional reconstruction of the right ICA shows the slippage of the previous straight clip. Reclipping via the use of an angled fenestrated clip, placed parallel to the long axis of the ICA, was accomplished.
Posterior ICA Wall Aneurysms: The Case for Coil Embolization
As mentioned above, many supraclinoid aneurysms are amenable to both endovascular and open treatment options. Despite its methodological shortcomings, the International Subarachnoid Aneurysm Trial (ISAT) can be generalized to many supraclinoid aneurysms. This trial demonstrated a statistical advantage in patient outcomes with endovascular treatment despite higher rates of recurrence and retreatment.
Most PCoA and some of the AChoA aneurysms are often amenable to conventional coiling techniques, sometimes requiring stent-assistance to preserve the ICA lumen, and sometimes requiring balloon-assistance, particularly if the branching vessel is involved with the aneurysm neck.
Preservation of the AChoA origin with coil embolization requires a precise working projection to ensure patency of the AChoA origin as this vital branch frequently arises from the neck of the aneurysm. Complete coiling of an aneurysm neck may be precluded. Compliant balloons can be useful in ensuring branch vessel patency by protecting the vessel origin when inflated beyond the diameter of the parent vessel.
Case Example 6
A 76-year-old woman presented with an incidental 9mm PCoA aneurysm and underwent effective coiling of the aneurysm. In the older patient with favorable aneurysm morphology, coiling is the preferred option.
Figure 6: Preoperative (L) and postoperative (R) angiograms demonstrate the effective exclusion of the aneurysm with preservation of the PCoA.
ICA Bifurcation Aneurysms: The Case for Microsurgical Clip Ligation
The ICA terminus aneurysms differ from other supraclinoid aneurysms in that numerous posteriorly and medially projecting perforators are intimately associated with the aneurysm neck; this fact increases the complexity and risk of microdissection, especially for large and giant aneurysms.
Typically the aneurysm dome is superiorly projecting and adherent to the orbitofrontal gyrus. With superiorly- or posteriorly-projecting aneurysms, visualization of the medial lenticulostriates may become obscured, whereas these vessels are more visible in the anteriorly-projecting aneurysms. The key is early identification of the M1, A1, and medial lenticulostriate arteries, including the ones emerging from the medial aneurysm neck wall.
Microsurgical clip ligation is a reasonable strategy for patients younger than 50 years of age. I do not hesitate to abort my microsurgical attempt and resort to endovascular therapy if preservation of small perforating vessels along the medial neck is found challenging intraoperatively for effective clip deployment.
Case Example 7
A 32 year-old female was found to harbor a large incidental ICA bifurcation and a small anterior choroidal artery aneurysm during work-up for headaches.
Figure 7: Clip ligation was the most durable option in this young patient to exclude the larger aneurysm and to attempt to also ligate the small aneurysm not amenable to endovascular intervention.
ICA Bifurcation Aneurysms: The Case for Endovascular Intervention
Internal carotid artery terminus aneurysms are often amenable to coil embolization with or without the assistance of a balloon or stent. These aneurysms can be treated endovascularly with minimal risk to the lenticulostriate arteries compared with open surgical treatment, particularly for superiorly and posteriorly-projecting aneurysms. Contrarily, anteriorly-projecting aneurysms may carry an angle that renders the microcatheter position slightly unstable. These lesions may be more amenable to clipping given their relative distance from the lenticulostriate arteries.
Flow-diverting stents covering the AChoA have led to few reported infarcts as long as there is sufficient adherence to the vessel contour. Given the low but real risk, covering the ACohA should be considered a disadvantage, but not a contraindication in a flow-diverting stent construct.
As already mentioned, dorsal wall blood blister-like aneurysms (BBLA) have an especially challenging disease process for both endovascular and open surgery. These aneurysms, which are essentially all ruptured aneurysms, own extremely fragile domes that can ruptured with either surgical or endovascular manipulation.
Although a thorough discussion of the risks and benefits of the options for this aneurysm subtype is beyond the scope of this chapter, suffice it to say that flow-diverting stents and bypass/trapping of the supraclinoid ICA have both been proven successful. Certainly the use of dual antiplatelet therapy needs to be carefully considered in the setting of rupture, especially in patients with acute hydrocephalus. I prefer endovascular therapy for these blister aneurysms as the first consideration.
Case Example 8
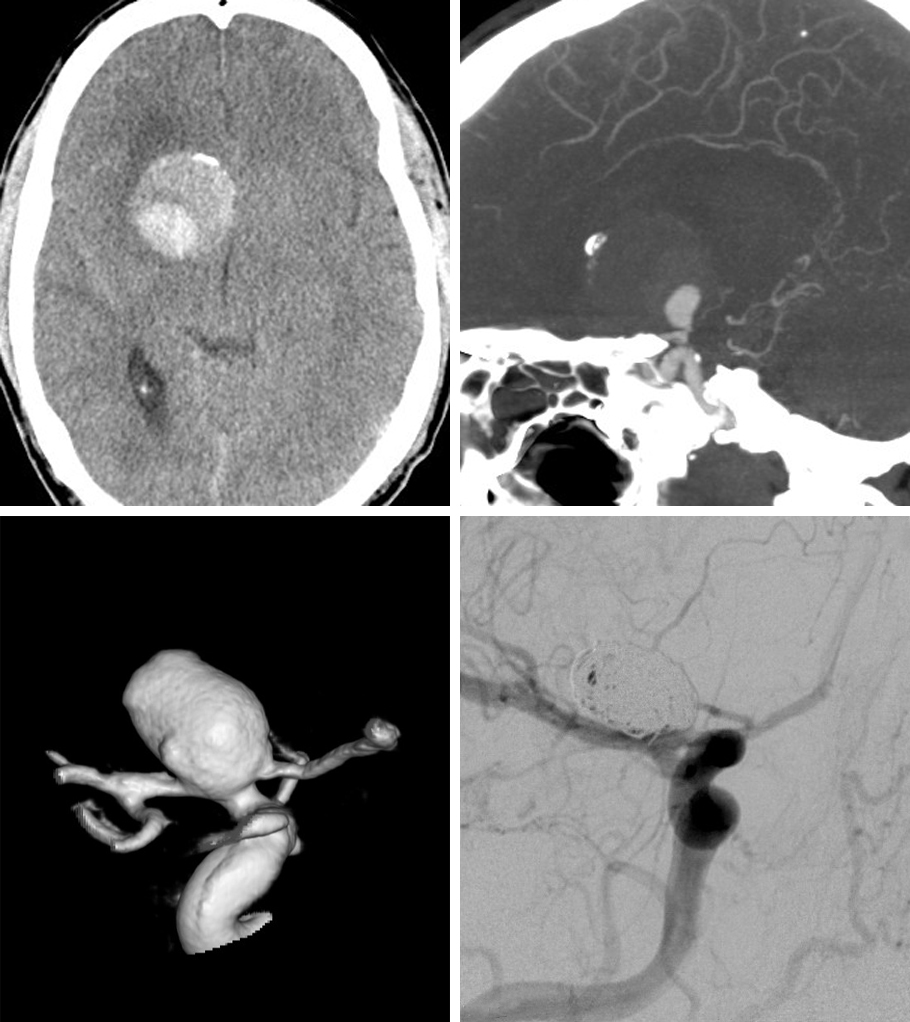
A 62 year-old male with a complicated medial history presented with headaches. Imaging demonstrated a large partially calcified and thrombotic aneurysm. Stent assisted coiling was chosen as calcification could have significantly increased the risks of microsurgical clipping.
Figure 8: Partial calcification and thrombosis of the aneurysm is apparent (top row). Stent-assisted coiling (bottom row) was accomplished uneventfully.
Case Example 9
A 67-year-old woman presented with progressive headaches and a family history of cerebral aneurysms. Her aneurysm faced slightly medially and away from the surgeon, making microsurgical exposure potentially difficult without excessive manipulation of the medial perforators.
Middle Cerebral Artery Aneurysms: The Case for Clip Ligation
Middle cerebral artery (MCA) aneurysms arise most commonly at the main bifurcation of the superior and inferior trunks, but they also may originate from the M1 at branching points of the lenticulostriate arteries, from early cortical branches, or distally along the branching points of the M2 or M3.
The technical difficulty of treating MCA aneurysms lies in identifying and mobilizing the arteries within the proximal and distal Sylvian cisterns. The anatomy may be confusing if there are multiple bridging or seemingly traversing vessels, but the key is to remember that arteries always remain faithful on the side of the lobe supplied.
This means that the same artery will supply only one lobe; therefore, arteries may be used to guide the laterality of Sylvian fissure dissection to avoid violating the pia. Veins, on the other hand, frequently bridge the Sylvian fissure and, therefore, may require coagulation and division. As a general rule, the larger superficial Sylvian veins should be maintained on the side of the temporal lobe.
A wide Sylvian fissure split is often necessary so that the surgeon can follow the opercular branches back to the insular branches and reach the main trunks. MCA aneurysms continue to be the most common indications for clip ligation because their relative superficial location facilitates atraumatic surgical exposure and clipping. Moreover, MCA aneurysms are also known to have broad necks, which make endovascular intervention more difficult and often requires assist devices.
An early anterior temporal branch or the inferior trunk may often be found adherent to the aneurysm sidewall and requires tedious dissection under temporary M1 clipping to enable segmentation and prevention of intraoperative rupture. Laterally-projecting MCA bifurcation aneurysms can obscure the inferior trunk; therefore, the back wall must be carefully dissected free for adequate clip placement. MCA aneurysms have a myriad of morphologies, requiring several practices ranging from simple or tandem clipping to clip reconstruction or bypass. These variations will be discussed in the corresponding chapter of this volume.
Case Example 10
A 58-year-old woman with a strong family history of intracranial aneurysms was found to have an incidental 5 mm M1 segment aneurysm. I elected clip ligation in order to preserve the small vital lenticulostriate perforators that arose from the neck of the aneurysm.
Figure 10: 3D angiogram (left) reveals a left-sided lentriculostriate artery aneurysm associated with a lentriculostriate artery by the origin of the aneurysm. Postoperative DSA shows the position of the angled fenestrated clip (right, inset) and total obliteration of the aneurysm with preservation of the lenticulostriate arteries (right).
Middle Cerebral Artery Aneurysms: The Case for Endovascular Intervention
The accessibility of MCAs is generally superior with open surgical techniques compared with endovascular methods. MCA aneurysms typically incorporate the origin of M2 or early M1 branches and often require coiling with either balloon or stent assistance.
Case Example 11
A 61-year-old man presented with SAH (Hunt-Hess grade 3). Based on the preference of the patient’s family and presenting neurological status, primary coil embolization of his medium-neck left MCA aneurysm was performed. After a number of attempts, a reasonably favorable placement of the framing coil was achieved.
Figure 11: Three-dimensional reconstruction of this MCA bifurcation aneurysm is shown (left) where the neck provided enough of a shelf to allow for primary coiling with some residual neck (right).
M1 segment aneurysms, contrarily, may be more favorable for endovascular treatment, particularly when the aneurysm projects posteriorly. Open surgical management of these aneurysms requires extensive dissection of the lenticulostriate perforators.
However, when the lenticulostriate branches arise from the neck of these aneurysms, coiling may have to be incomplete to preserve the eloquent vessels. Endovascular treatment of distal MCA aneurysms is rarely the more favorable option due to difficulties with accessing distal branches and preserving the lumen of associated small-caliber vessels. Some aneurysms may be amenable to coil embolization, and the introduction of stents deployable through 0.017” microcatheters may facilitate parent vessel preservation and aneurysm obliteration.
Parent vessel occlusion can be a viable option in rare cases when both clipping and coiling treatment options are inadequate. Microcatheter amytal testing or balloon test occlusion prior to definitive parent vessel occlusion can help determine the safety of this strategy, which may be more desirable for patients with infectious or mycotic aneurysms.
Case Example 12
A 57-year-old man presented with an incidental left large calcified MCA aneurysm, discovered during diagnostic tests for chest pain and right arm numbness. The aneurysm was initially treated endovascularly in an outside facility. After 1 year, a recurrence was detected at the aneurysm neck. Further coiling was performed which provided Raymond 1 obliteration.
Figure 12: A multilobulated calcified aneurysm underwent coiling due to significant calcification at the neck. Post-coiling DSA showed uneventful obliteration of the aneurysm with preservation of the neighboring branches (top row). Follow-up DSA revealed a recurrence at the neck, requiring recoiling; final angiogram shows Raymond grade 1 obliteration (bottom row).
Case Example 13
An 85 year-old male suffered from SAH and underwent “palliative” coiling of his giant MCA aneurysm. Repeat coiling was necessary 2 years later. He has remained neurologically intact. Clipping and revascularization was deemed too risky for this patient.
Figure 13: This giant aneurysm underwent palliative coiling in this elderly patient to avoid a high-risk microsurgical procedure requiring bypass.
Anterior Cerebral Artery Aneurysms: The Case for Clipping
Anterior communicating artery (ACoA) aneurysm surgery is more complicated than MCA aneurysm surgery because dissection requires potential identification and preservation of at least 11 arteries. These include the bilateral A1 and A2 segments, the ACoA, bilateral recurrent arteries of heubner, orbitofrontal, and frontopolar arteries.
The ACoA perforators are also numerous and arise from the superior aspect of the A1 segment and the posterosuperior aspect of the ACoA. Rarely these perforators may also stem from the anterior and inferior aspects of the ACoA traveling to supply the optic chiasm.
The need for subfrontal retraction, gyrus rectus resection and manipulation of the ACoA perforators carries a significant risk of neurocognitive decline. In high-functioning individuals, these maneuvers can significantly affect their quality of life. This phenomenon is especially true for superiorly and posteriorly projecting aneurysms.
Anteriorly-projecting ACoA aneurysms may obscure the contralateral A1-A2 junction and the origin of the recurrent artery of Heubner. Inferiorly-projecting aneurysms may obscure the contralateral A1 segment, whereas a superior projection obscures the contralateral A2 segment.
Posteriorly-projecting ACoA aneurysms are the most difficult to treat microsurgically because the aneurysm neck is intertwined between the numerous ACoA perforators. High-riding ACoA, swollen “subarachnoid” brain, or dense adhesions limit the standard exposure and necessitate more extensive subpial dissection, transection of the gyrus rectus, or an orbitotomy. These factors should lead the operator to strongly consider endovascular therapy.
Treatment of distal ACA or pericallosal artery aneurysms should be determined based on their location. The morphology of the aneurysm neck compared to the parent vessel determines the suitability of endovascular therapy.
Case Example 14
A 45-year-old woman presented with a recurrent SAH secondary to a small ACoA aneurysm. Three years earlier, she had undergone coiling of the aneurysm in another hospital. After the rehemorrhage, she underwent microsurgical clipping.
Figure 14: Angiography performed at the time of the initial coiling is shown (top, left). The patient presented three year later with recurrent SAH and persistent minimal residual filling of the aneurysm. Intraoperative picture of the aneurysm before (middle, left) and after clipping (middle, right) are included. Postoperative angiography confirmed complete obliteration of the aneurysm (bottom images).
Anterior Cerebral Artery Aneurysms: The Case for Coiling
Anterior communicating artery aneurysms that are potential candidates for endovascular treatment fall into one of two categories: ACoA or distal ACA aneurysms. Endovascular treatment of ACoA aneurysms is favorable particularly for posteriorly and superiorly-projecting aneurysms, which are less safely accessible from an open surgical approach. “High-riding” ACoA aneurysms within the interhemispheric fissure might also lead the operator to tend toward endovascular treatment.
These aneurysms often incorporate the parent vessels into their neck, and various stenting strategies can be used to preserve the parent and branching vessels including Y-stenting, crossing stents, stents from ipsilateral A2 to ipsilateral A1, and stents from contralateral A2 to ipsilateral A1. Balloon assistance may also be appropriate in patients suffering from subarachnoid hemorrhage and ventricular drainage.
Distal ACA aneurysms can be reached microsurgically from an interhemispheric approach; access may be more limited endovascularly. Although endovascular technology has improved to allow stenting in small distal vessels, there are still some technical challenges and results may not support endovascular treatment as a primary option over surgical clipping.
Case Example 15
A 59-year-old man presented with a sudden onset sentinel headache and was diagnosed with an ACoA aneurysm. The wide neck of the aneurysm necessitated balloon-assisted coiling. Total occlusion was achieved.
Figure 15: A right ICA angiogram reveals a small, broad-based ACoA aneurysm (left). Postoperative imaging confirms adequate obliteration (right).
Contributors: Christopher Baggott, MD, Ulas Cikla, MD, Clemens M. Schirmer, MD, PhD, and Mustafa K. Baskaya, MD
References
Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30:470-476.
David CA, Vishteh AG, Spetzler RF, et al. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999;91:396-394.
Elsharkawy A, Lehecka M, Niemela M, et al. A new, more accurate classification of middle cerebral artery aneurysms: computed tomography angiographic study of 1,009 consecutive cases with 1,309 middle cerebral artery aneurysms. Neurosurgery. 2013;73:94-102.
Hayakawa M, Murayama Y, Duckwiler GR, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561-568.
King JT, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg. 1994;81:837-842.
Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg. 2003;98:959-966.
Raaymakers TW, Rinkel GJ, Limburg M, Algra A. Mortality and mobidity of surgery for unruptured intracranial aneurysms: a meta-analysis. Stroke. 1998;29:1531-1538.
Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T. Risk of subarachnoid hemorrhage after surgical treatment of unruptured cerebral aneurysms. Stroke. 1999;30:1181-1184.
Please login to post a comment.