Intraparenchymal Hematoma
Historically, the surgical management of intraparenchymal hemorrhage (IPH) has been heavily controversial within the literature. IPH has been described since the days of Hippocrates, and Avicenna alludes to it as well in his famous text. Not until far later were we presented with the first description of successful operative evacuation of spontaneous IPHs, for which we are indebted to Sir William MacEwen and Harvey Cushing.
In subsequent years, several small monographs were published on the subject. The first major series published by McKissock and colleagues in 1961 showed no added advantage of surgical management for IPH over other treatments. However, in the pre-CT and pre-microscope era in which the research was conducted, we can wonder to what extent the results were impacted by the lack of tools that investigators today find crucial to successful neurosurgery and patient care.
Subsequent trials were also inconclusive until the STICH (Surgical Trial in Traumatic Intracerebral Hemorrhage) trial, followed by the STICH II (Surgical Trial in Lobar Intracerebral Hemorrhage) trials, were published in 2005 and 2013, respectively. Results from the STICH trial suggested favorable outcomes in the setting of hematoma at or less than 1 cm from the cortical surface. However, no significant benefit was noted in functional outcomes for early surgery or for deep basal ganglia hemorrhages compared with medical management.
Endoscopic surgery is now being evaluated for evacuation of deep hematomas, allowing minimal disruption of normal brain tissue, which was thought to be the shortcoming of evacuating hematomas within the deep nuclei and farther than 1 cm from the cortical surface. Favorable outcomes have been demonstrated in several studies. Cho and colleagues compared endoscopic, stereotactic, and open craniotomy methods for hematoma evacuation and found that the endoscopic approach was associated with the highest rate of hematoma evacuation and the lowest rate of mortality and complications.
Introduction
According to CDC data, 795,000 people annually in the United States have a stroke, of which 87% are ischemic and 13% are hemorrhagic. Hemorrhagic strokes are distinct and require significantly different medical and surgical management. The mechanical clot burden and risk to ischemic penumbra is a driving principle in determining if surgical evacuation has a role in the treatment of hemorrhagic stroke.
Hemorrhagic stroke, otherwise known as spontaneous IPH, is one of two major subdivisions of IPH, the other being traumatic. The differential diagnosis for a spontaneous IPH includes atherosclerotic hypertensive cerebrovascular disease, cerebral amyloid angiopathy, central nervous system (CNS) vasculitides, hemorrhage into an underlying brain tumor (most commonly metastatic lesions: lung, renal, melanoma), CNS infection (herpes encephalitis), cavernous malformations, arteriovenous malformations, mycotic aneurysms, Moyamoya disease, and dural arteriovenous fistulae.
Coagulopathy, or thrombocytopenia, can also be the cause of or contributor to spontaneous IPH. Overall, the most common etiology of spontaneous IPH is hypertensive vasculopathy. Common locations for hypertensive hemorrhages include putaminal, lobar, thalamic, cerebellar, and pontine. This chapter will focus on the surgical management of spontaneous and traumatic IPH.
Presentation
Patient presentation is dependent on the region of parenchyma affected by the hemorrhage. Common presenting symptoms include hemiparesis/plegia, hemianesthesia, facial droop, dysarthria, and dys/aphasia. An altered mental status can be part of this constellation if there is significant brain compression from midline shift or intraventricular extension of the clot.
In the setting of trauma, IPH can be incidentally discovered on computed tomography (CT) of the head, or it can present with a spectrum of neurologic states ranging from concussed to comatose. These traumatic contusions have a propensity to blossom in a delayed fashion and potentially cause significant increases in intracranial pressure, requiring intensive medical and possibly surgical management. Similarly, in the setting of stroke, the degree of cerebral edema impacts the patient’s level of consciousness.
The ICH score is an excellent parameter designed by Hemphill and colleagues at UCSF to predict 30-day mortality and can therefore be a stratification tool when deciding whether the patient’s condition warrants surgical intervention.
| 0 | +1 | +2 | |
| GCS | 13-15 | 5-12 | 3-4 |
| Age | <80 years | >=80 years | - |
| ICH Volume | <30cm3 | >=30cm3 | - |
| Intraventricular hemorrhage | No | Yes | - |
| Infratentorial origin of ICH | No | Yes | - |
|
ICH Score 0 = no mortality ICH Score 1 = 13% ICH Score 2 = 26% ICH Score 3 = 72% ICH Score 4 = 97% ICH Score 5 = 100% ICH Score 6 = 100% |
|||
Diagnosis
In keeping with the standard of care for any patient beset with signs of stroke or traumatic brain injury, it is crucial to obtain a head CT scan without contrast. CT angiography (CTA) of the head and neck can be used to evaluate the cranial vasculature in the setting of stroke for possible underlying vascular anomaly, vascular injury, aneurysm, dissection, or thrombus.
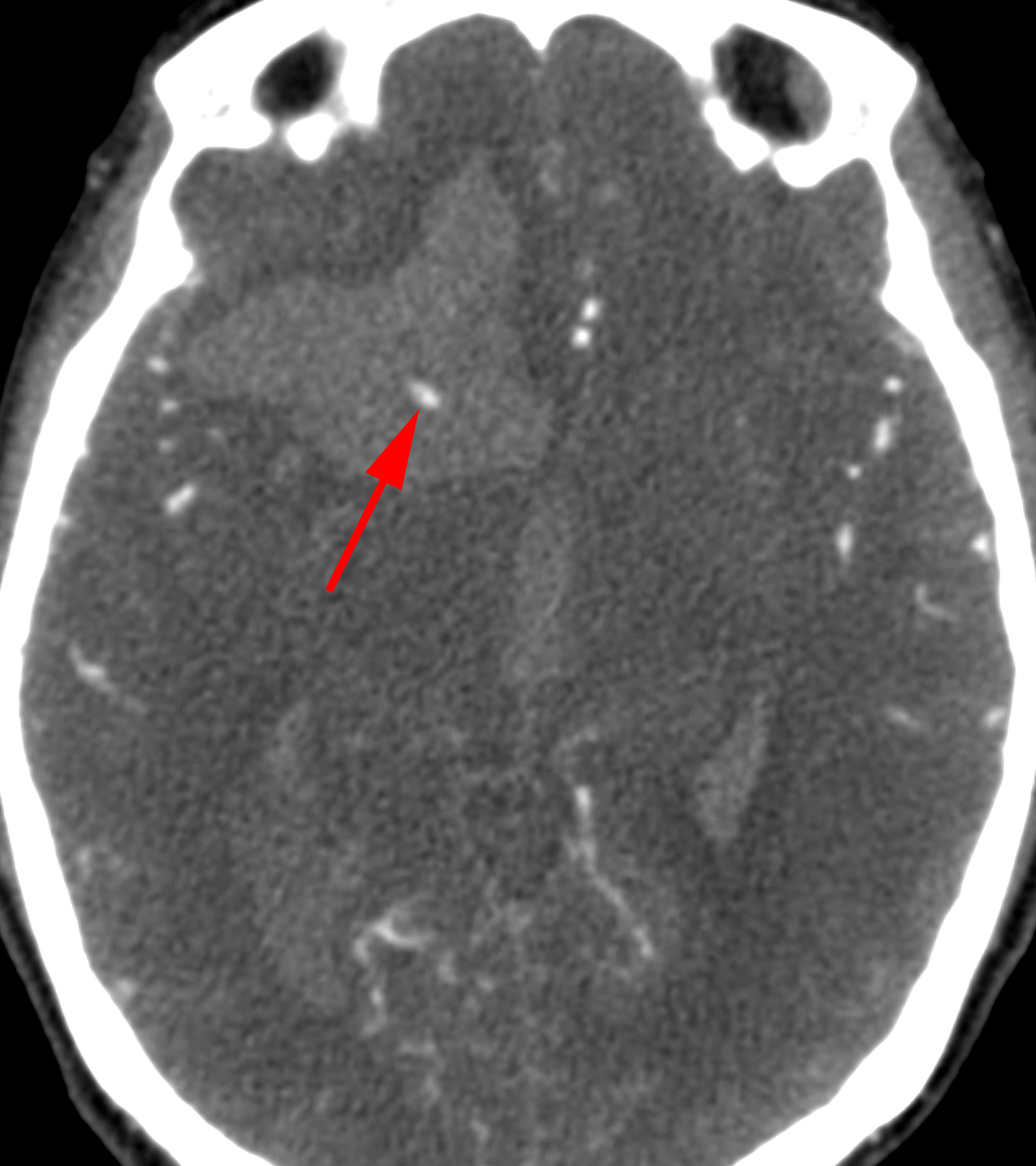
On a noncontrasted CT head, IPH or contusion will appear as a hyperdense, commonly concentric or ovoid mass with or without surrounding edema. On the CTA, special attention should be given to identify a “spot sign” within the hematoma, which indicates active, dynamic hemorrhage and is predictive of hematoma expansion, intraoperative bleeding and worse outcomes for the patient.
Figure 1: The “spot sign” is apparent in the middle of the hematoma cavity, indicative of active bleeding and extravasation of contrast. This sign refers to unifocal or multifocal contrast enhancement within an acute hemorrhage visible on CTA source images and discontinuous from adjacent normal or abnormal blood vessels.
It is important to evaluate for the presence of midline shift, basal cistern effacement, and caudal temporal/cerebellar displacement suggestive of herniation—an affliction for which urgent decompression may be required. The ventricular system should be analyzed for hydrocephalus, which, if present, warrants placement of an external ventricular drain (EVD).
In the acute care setting of hemorrhagic stroke or traumatic IPH, contrasted brain magnetic resonance (MR) imaging does not change management but can be helpful once the patient is stabilized if there is suspicion of an underlying lesion.
Indications for Surgery
Supratentorial Hematoma
Unlike epidural and subdural hematomas, there are no set indications for operative intervention for IPH and surgical decision making is more dependent on the patient’s presenting GCS, hematoma size on CT (higher rate of progression and mortality if clot > 30 cc), age, co-morbidities, and preoperative neurological deficits.
Lobar hematomas within 1 cm of the cortical surface are most amenable to surgical intervention. If there is worsening of GCS and CT head reveals correlative increase in size of the hematoma, perilesional edema, compression of basal cistern or early signs of transtentorial herniation, surgery should be considered. Surgery for basal ganglia hematomas is still controversial, with an increasing number of studies showing promise with endoscopic and stereotactic approaches.
Infratentorial Hematoma
Cerebellar hematomas, if presenting with GCS < or = 13 or hematoma size > 3 cm should be evacuated as early as possible given the potential irreversible deterioration that may occur within minutes to hours. Early hydrocephalus from cerebellar hematomas should also compel the surgeon to urgently evacuate the hematoma. Placement of an EVD should be considered if hydrocephalus is visible on the initial CT head but should not delay prompt surgical decompression.
Figure 2: Although removal of 3 cm cerebellar hematomas should be strongly considered, the resultant mass effect can be variable in different patients. In the above scan, the hematoma was 4 cm in an awake patient. Observation was pursued due to the patient’s neurological exam and an absence of significant mass effect.
Preoperative Considerations
In the preoperative setting, additional diagnostic studies are necessary. The patient’s prothrombin time (PT), partial thromboplastin time (PTT), and international normalized ratio (INR) are assessed. Alternatively, a thromboelastogram evaluation can provide functional analysis of coagulation status and platelet function. The use of antiplatelet and anticoagulation medications should be reviewed.
Correction of coagulation and platelet dysfunctions prior to surgery should be considered. Administration of platelets to reverse the effect of anti-platelet therapy has been challenged by the PATCH (Platelet Transfusion versus Standard Care after Acute Stroke due to Spontaneous Cerebral hemorrhage associated with Antiplatelet Therapy) trial recently published in the Lancet. However, in the setting of surgery, especially with use of clopidogrel, it is imperative to reverse the effects by means of platelet transfusion. As we more commonly encounter the use of novel anticoagulant agents, knowledge of the appropriate reversal mechanism is essential.
In the setting of a major change in neurologic status or immediately prior to surgical decompression, a head CT should be obtained to assess the current status of the hemorrhage—a necessary action given the potential for rapid change in the hematoma configuration. If increasing intracranial pressure is responsible for the patient’s neurologic deterioration, appropriate preoperative measures should be instituted, including the following:
- head elevation
- hyperosmolar therapy (0.5-1 gm/kg of mannitol or hypertonic saline bolus)
- EVD placement
- hyperventilation (PCO2 of 30-35 mmHg)
If, in cases of trauma, the patient is wearing a cervical collar, the collar should be loosened to prevent venous outflow obstruction.
An arterial line should be inserted to monitor arterial systolic blood pressure. While there is evidence to support SBP of 150 or less to prevent progression of hemorrhage in the acute ictus phase, the literature offers no clear evidence of blood pressure parameters to maintain in the setting of traumatic IPH. An arterial line is also helpful to monitor blood pressure during induction of anesthesia and intubation prior to surgery, when blood pressures can be labile.
Prophylactic antiepileptic drugs should be strongly considered, particularly in patients with lobar hemorrhage as there is 7-10% risk of seizures in the first few days of the ictus.
SURGICAL EVACUATION OF INTRAPARENCHYMAL HEMATOMA
Supratentorial
A craniotomy for supratentorial lobar hematomas is a straightforward procedure and can involve a craniotomy centered over the region of the parenchyma housing the hematoma. General technical principles that should be considered are as follows:
- the corticotomy should be performed where the hematoma is closest to the cortical surface (usually less than 1 cm in depth)
- avoid regions of eloquent cortex
- avoid transgressing capsular fibers deep to the hematoma cavity
For hemorrhages within the deep nuclei, a transsylvian-transinsular route is commonly the least invasive method to access the clot. Endoscopic techniques employ a supraorbital craniotomy through the frontal lobe to reach the hematoma along its long axis.
Head positioning is based on the site of the hematoma. For frontopolar hematomas, a supine position on a horseshoe headrest is adequate. For more posterior hematomas, the head can be turned contralateral to the lesion, with a gel roll placed under the ipsilateral shoulder to facilitate rotation of the neck.
Once the head is secured, the incision is planned, correlating anatomical landmarks on the scalp (pinna, coronal suture, etc.); neuronavigation-based image guidance may be used, if available. A linear or curvilinear incision can be employed for most craniotomies. A small trauma flap can also be utilized for low frontotemporal or large-volume hemispheric hematomas. If it is suspected that a craniectomy may be required based on the lesion volume or the degree of cerebral edema on preoperative imaging, then a large trauma flap should be performed for appropriate decompression.
Once the craniotomy is complete and the bone flap elevated, the dura is often under significant tension from the underlying edematous parenchyma. Dural incision should be well thought out to prevent massive brain herniation during initial dural opening. Administration of mannitol with furosemide should be considered at the time of intubation to facilitate brain relaxation.
Initially, I complete a small cruciate dural opening directly over the region where the clot volume is closest to the cortical surface. A small corticotomy is made using bipolar electrocautery and suction to enter the hematoma cavity. The hematoma is of gel-like consistency and delivers quite easily via suction aspiration and gentle maneuvering using a dissector or a pair of bipolar forceps. Gentle irrigation can also be helpful in freeing the hematoma from the walls of the cavity.
Care should be taken to not enter the walls of the cavity lined by the white matter tracts to prevent added neurologic injury. A cottonoid can line the cavity wall so that dynamic retraction using the suction device can be employed, allowing for inspection within the cavity for active bleeding. I usually use the operating room microscope or an endoscope.
An active hemorrhage is uncommonly found unless the walls of the cavity are transgressed inadvertently. If active bleeding is present, it can be controlled with bipolar electrocautery. Venous oozing is handled via patience and gentle irrigation as well as application of oxidized cellulose polymer (Surgicel®, Ethicon US LLC). I prefer the use of thrombin irrigation. Aggressive bipolar coagulation of the regions demonstrating venous oozing can significantly worsen the bleeding and lead to white matter injury.
I do not pursue small deep hematomas to minimize the risk of injury. Once adequate removal of the clot has been achieved, the visible parenchyma within the surgical field is inspected. If it appears relaxed, the bone flap should be prepared for replacement.
If continued cortical tension is encountered, the dural flaps should be enlarged widely to complete a duraplasty. An EVD or intracranial pressure monitor should also be considered to allow drainage of cerebrospinal fluid and pressure monitoring. In such a situation, it would also be prudent to obtain a CT head immediately postoperatively to ensure absence of new hemorrhage or infarct and appropriate EVD placement.
In the setting of a traumatic hemorrhage, surface contusions can be present on the convexity of the brain. These are usually left undisturbed unless there is significant mass effect from them.
Infratentorial
A standard midline suboccipital craniotomy is then performed to expose cerebellar hemorrhages. A craniectomy should be considered if significant cerebellar swelling is expected despite decompression; otherwise, a simple craniotomy with replacement of the bone flap or fixation of mesh cranioplasty is adequate.
The patient’s head is immobilized in a Mayfield skull clamp and the body is positioned prone on the operating room table. Alternatives based on the surgeon’s preference include the use of a lateral or park-bench positions.
Gentle neck flexion and chin tuck elevate the suboccipital region. Frazier’s point can be incorporated into the sterile draped area to allow placement of an occipital horn ventricular drain, if needed. Special care should be taken to avoid hyperflexion, which can increase jugular venous pressure and lead to raised intracranial pressure.
A midline or paramedian incision, usually made from inion to C2, is planned based on the site of hemorrhage. The avascular midline should be entered to avoid excessive cervical muscular bleeding and postoperative pain.
Lateral dissection over the posterior arch of C1 should be cautiously performed at lower cautery settings. During this step, blunt dissection is advised to avoid injury to the vertebral arteries. These arteries can have a very tortuous route outside their groove over the lateral arch of C1 (sulcus arteriosus) and may travel within the suboccipital muscles. Indiscriminate use of cautery can lead to vascular injury. Brisk bleeding from the venous plexus encasing the artery is a warning sign that the artery is nearby.
Dissection below the inferior lip of C1 lamina is not necessary. Muscular attachments on C2 should be left intact.
Once the suboccipital region is exposed, two burr holes are placed on either side of the midline keel. The bone is thin there, and therefore minimal drilling is required. The craniotomy is completed, which can be eccentric toward the ipsilateral hemorrhage. The midline keel can also be removed using larger rongeurs. Major dural venous sinuses are not unroofed.
The dura is usually opened in a Y-shaped fashion to allow easier dural closure at the end of the case. A small corticectomy may be required over the cerebellar hemisphere through which the hematoma is delivered. The hematoma cavity is inspected thoroughly to ensure the absence of an occult vascular anomaly that was not seen on the CTA. Care should be taken to prevent injury to the distal posterior cerebellar arteries given their close proximity.
The deep midline perforating cerebellar arteries that lead to hypertensive hemorrhages can bleed profusely during hematoma evacuation. Securing hemostasis patiently is the key.
Complication Management
Management of intracranial pressure is paramount during surgical evacuation of an IPH. Significant cerebral edema can precipitate brain herniation following dural opening; therefore, the surgeon should remain cognizant of the augmentative measures for lowering ICP that can be performed by the anesthesiologist. Additionally, gentle compression on the brain through the dural defect with a saline-soaked sponge can be applied while these maneuvers are being instituted. The positive effects of head elevation in these situations are often underestimated.
Bolus CSF drainage though the external ventricular drain is also an option both perioperatively and intraoperatively. If the edema is not controlled with these conservative maneuvers, then a contralateral frontal EVD can be placed for additional drainage. If the Frazier’s point was incorporated into the sterile draping, an occipital horn EVD is an option. Placement of additional drains should be considered once the hematoma has been evacuated.
In the setting of extensive cerebral edema after evacuation of the hematoma, the bone flap should not be replaced to allow continued parenchymal decompression.
Closure
If the parenchyma is relaxed at the end of the procedure, the dura is approximated with nylon suture and the bone flap is replaced followed by standard skin closure. A piece of pericranium or synthetic dural substitutes is used if needed.
I do not pursue watertight dural closure in most cases. If brain edema is encountered, the dural leaflets are loosely reflected back and the dural closure continues by means of a piece of pericranial flap or synthetic dura.
Postoperative Considerations
Most patients will remain intubated and return to the ICU. An arterial line is used to maintain the systolic blood pressure to less than 150 mmHg. A postoperative CT scan should be obtained early to exclude rehemorrhage or remote bleeding. Hourly neurological checks should be instituted, and once a baseline is determined, any unexplained alterations should prompt repeat imaging.
Appropriate laboratory studies should be drawn to check for an unexpected postoperative coagulopathy that may occur due to release of tissue thromboplastins. Goals of care should also be discussed with the immediate family to determine the plan of further care.
Pearls and Pitfalls
- The surgeon should be prepared to encounter a vascular anomaly that may not have been detected on preoperative vascular imaging.
- The size of the corticotomy should be minimized, and the white matter tracts in the walls of the cavity should be respected to avoid further injury. Too small of corticotomy can lead to suboptimal hematoma evacuation.
Contributor: Farhan A. Mirza, MBBS, Benjamin K. Hendricks, MD
References
Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70(4):530-535.
Baharoglu MI,Cordonnier C,Al-Shahi Salman R,de Gans K,Koopman MM,Brand A,et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387(10038):2605–2613.
Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006;65:547-555. 10.1016/j.surneu.2005.09.032. discussion 555–546.
Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891-897.
Kim YZ, Kim KH. Even in patients with a small hemorrhagic volume, stereotactic-guided evacuation of spontaneous intracerebral hemorrhage improves functional outcome. J Korean Neurosurg Soc. 2009;46:109-115. 10.3340/jkns.2009.46.2.109.
Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. 2012; 29(1):19–31.
Li Y, Zhang H, Wang X, She L, Yan Z, Zhang N, et al. Neuroendoscopic surgery versus external ventricular drainage alone or with intraventricular fibrinolysis for intraventricular hemorrhage secondary to spontaneous supratentorial hemorrhage: a systematic review and meta-analysis. PLoS One. 2013;8(11):e80599. doi: 10.1371/journal.pone.0080599. eCollection 2013.
Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387-397.
Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397-408. Erratum in: Lancet. 2013;382(9890):396.
Mocco J. Minimally Invasive Endoscopic Surgical Treatment With Apollo in Patients With Brain Hemorrhage. https://clinicaltrials.gov/ct2/show/NCT02661672. Accessed 9 September 2017.
Nakano T, Ohkuma H. Surgery versus conservative treatment for intracerebral haemorrhage--is there an end to the long controversy? Lancet. 2005;365(9457):361-362.
Park SY, Kong MH, Kim JH, Kang DS, Song KY, Huh SK. Role of “spot sign” on CT angiography to predict hematoma expansion in spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc. 2010;48(5):399–405.
Albright AL, Pollack IF, Adelson PD (Eds). Principles and Practice of Pediatric Neurosurgery. 3rd ed. New York: Thieme, 2014.
Qureshi AI, Mendelow AD, Hanley DF. Intracerebral hemorrhage. Lancet. 2009;373(9675):1632–1644.
Winn H (Ed). Youmans Neurological Surgery, 6th ed. Philadelphia: Saunders, 2011.
Zan X,Li H,Liu W,Fang Y,Ma J,Lan Z,et al. Endoscopic surgery versus conservative treatment for the moderate-volume hematoma in spontaneous basal ganglia hemorrhage (ECMOH): study protocol for a randomized controlled trial. BMC Neurology. 2012;12:34. DOI: 10.1186/1471-2377-12-34.
Please login to post a comment.