Imaging Evaluation of SAH and Aneurysm Free
Overview
- Subarachnoid hemorrhage (SAH)
- Ruptured intracranial aneurysm (80% of nontraumatic cases)
- Vascular malformations (up to 5% of cases)
- Imaging workup
- Nonenhanced CT (NECT) of the head (sensitivity 98%)
- CTA
- Performed if NECT results are positive
- Specificity 100%, sensitivity 96% to 99.7% for >4-mm aneurysms
- MRI/MRA
- Uncommonly used for acute SAH
- For more information, please see the corresponding chapter in Radiopaedia
- Intracranial aneurysms
- Incidence of intracranial aneurysms is 2% to 3% of the general population
- Saccular aneurysms
- Typically arise at arterial branch points
- Far more common than intracranial arterial dissections and dissecting pseudoaneurysms
- May be associated with high-velocity flow phenomena
- Feeding vessels to dural arteriovenous fistulas (AVFs)
- Feeding and intranidal arteriovenous malformation (AVM) vessels
- Vessels associated with infection, trauma, and neoplasm
- Cause <5% of all aneurysms
- Acute onset of severe headache is the typical presentation indicating aneurysm rupture
- Progressive sequelae
- Acute hydrocephalus
- Vasospasm
- Cerebral edema
- Ischemic infarct
- Herniation
- Coma
- Death
-
For more information, please see the corresponding chapter in Radiopaedia
- Imaging workup
- CTA superior to MRI for detection
- MRI/MRA for surveillance of unruptured/treated aneurysms
- DSA
- Gold standard for evaluation of aneurysms detected by cross-sectional angiography and for preoperative and postoperative characterization
- Enables endovascular access for embolization
- Rotational angiography using C-arm greatly enhances spatial and temporal resolution compared to CT
Imaging
Nonenhanced Computed Tomography
- Technique
- Axial 5-mm collimation is typical
- If acquired helically, reformatted images in coronal/sagittal planes can be made available in difficult cases, although they are generally not needed
- Findings
- Hyperdense (50–75 Hounsfield units [HU]) foci or collections in sulcal and/or cisternal subarachnoid spaces representing SAH
- High sensitivity of detection (>75%)
- Variable appearance can suggest aneurysm location, with SAH often thickest near the site of rupture
- Interhemispheric cisterns (anterior communicating artery [AComA] aneurysms)
- Suprasellar cistern (internal carotid-posterior communicating artery [IC-PcomA], AComA aneurysms)
- Sylvian fissure (middle cerebral artery [MCA] bifurcation aneurysms)
- Prepontine, cerebellopontine cisterns, fourth ventricle (posterior inferior cerebellar artery [PICA], basilar artery [BA], vertebral artery [VA] aneurysms)
- Perimesencephalic SAH (see DDx)
- Distinct pattern of SAH
- Centered on the basal cisterns around the midbrain, interpeduncular cistern
-
For more information, please see the corresponding chapter in Radiopaedia
-
See Figures 1 and 2
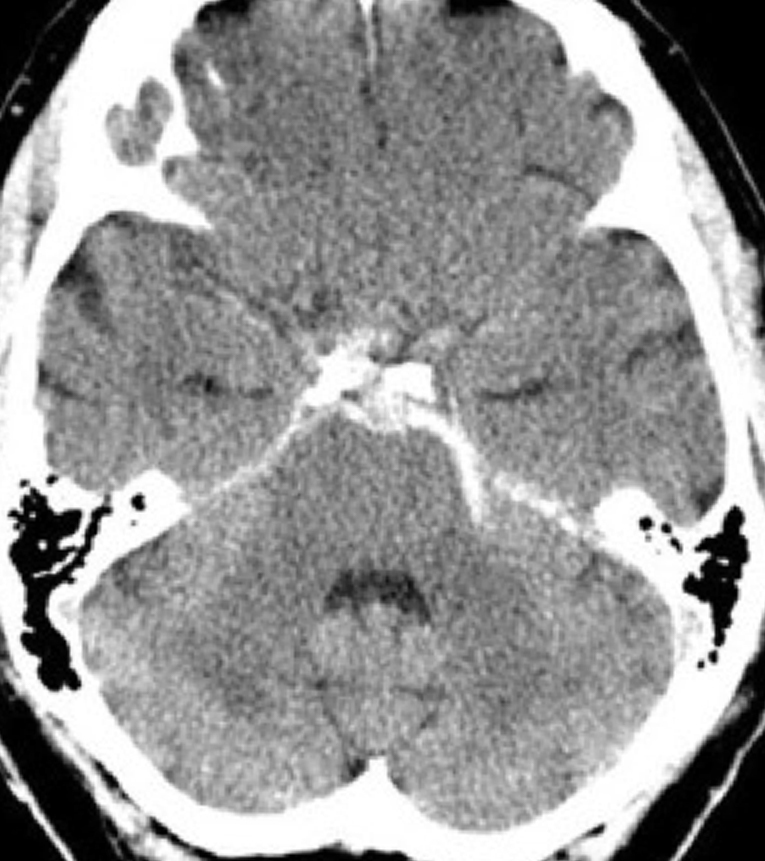
- Fisher CT grading in SAH
- 1—no SAH visible
- 2—diffuse, thin layer (<1 mm)
- 3—localized clot or thick layer (>1 mm)
- 4—intraventricular blood
- See Figure 3
- Detectable sequelae of SAH
- Intraventricular hemorrhage
- Hydrocephalus (can be seen at initial presentation, up to 90%)
- Cerebral edema
- Ischemic infarct
- Mass effect
- Herniation
- Terson syndrome
- Vitreous hemorrhage seen in up 13% of SAH cases
- Associated with severe SAH
- Hyperdense (50–75 Hounsfield units [HU]) foci or collections in sulcal and/or cisternal subarachnoid spaces representing SAH
Figure 1: Perimesencephalic SAH. Axial NECT image of the head demonstrates hyperdense blood products layering within the perimesencephalic cisterns, consistent with SAH (Fisher grade 2).
Figure 2: Perimesencephalic SAH. Axial NECT image of the head demonstrates hyperdense blood products layering within the perimesencephalic cisterns eccentric to the left, consistent with SAH (Fisher grade 2).
Figure 3: Nonperimesencephalic SAH. Axial NECT image of the head demonstrates hyperdense blood products layering within the suprasellar cisterns anterior to the midbrain and expanding into the sylvian cisterns consistent with SAH (Fisher grade 3). Note the enlargement of the temporal horns indicating developing hydrocephalus.
- Limitations
- Insensitive for detection of the aneurysm itself given lack of contrast and collimation, although large and giant aneurysms and resultant mass effect can be detected
- Thrombosed aneurysms may be identifiable, appearing hyperdense to parenchyma
- Aneurysm may demonstrate mural calcifications
- Beam hardening artifact obscuring SAH
- Bone (skull base and posterior fossa)
- External or foreign body metallic artifact
- High attenuation of previously treated aneurysms by endovascular coil mass or aneurysm clips
- Insensitive for detection of the aneurysm itself given lack of contrast and collimation, although large and giant aneurysms and resultant mass effect can be detected
- DDx
- AVM or AVF, intracranial and cervical spine
- Nonaneurysmal perimesencephalic SAH
- Potentially venous origin
- Diagnosis of exclusion
- Intracranial arterial dissection
- Traumatic SAH
-
For more information, please see the corresponding chapter in Radiopaedia
-
- Cavernous malformation
- Vasculitis or other vasculopathy
- Leptomeningeal metastasis, hemorrhagic metastasis
- Leptomeningeal infection (meningitis), infectious (mycotic) aneurysm
- Diffuse cerebral edema (pseudo-SAH sign)
- Diffuse hypoattenuation of the brain parenchyma makes normal arteries look hyperdense, can mimic SAH
-
For more information, please see the corresponding chapter in Radiopaedia
-
See Figure 4
- Reversible cerebral vasoconstriction syndrome (RCVS)
- Recent intrathecal contrast administration
- Subarachnoid leakage of recent intra-arterial contrast injection, often in area of recent infarct
- See Figure 5
Figure 4: Pseudo-SAH sign. Axial NECT images of the head of 2 different patients demonstrate diffuse cerebral edema indicated by diffuse hypoattenuation of the brain parenchyma. Note the relative hyperdensity of the normal cerebral arteries within the subarachnoid space, mimicking SAH.
Figure 5: Contrast extravasation. Axial NECT image of the head demonstrates leakage of contrast into the subarachnoid space after intra-arterial contrast injection for angiography and thrombectomy due to increased vascular permeability from recent completed infarct.
Computed Tomography Angiography
- Technique
- Thin collimation (1–2 mm) is typical
- Reconstructions
- Reformatted imaging in coronal/sagittal planes
- Maximum intensity projection (10-mm slabs)
- Volumetric reconstructions
- Iodinated contrast injection rates of 4 to 5 mL/sec of highly concentrated medium (iodine, 350–370 mmol/mL) are preferable
- Can be done concomitantly with NECT
- Findings
- Patent aneurysms typically demonstrate uniform postcontrast enhancement
- High sensitivity for aneurysm detection
- 90% to 95% sensitive for aneurysms ≥2 mm
- 96% to 99% sensitive for aneurysms ≥4 mm
- High specificity (~100%)
- CTA is superior to MRA for detection and overall characterization of saccular aneurysm geometry
- May detect dissecting aneurysm
- Demonstration of active extravasation
- Focal enhancement within area of hemorrhage or hematoma (“spot sign,” rare)
- Limitations
- Beam hardening artifact
- Bone (skull base and posterior fossa)
- Can obscure PComA and AComA aneurysms as well as posterior circulation aneurysms
- Dual-energy CT has promise in improving detection of aneurysms in these locations, because threshold HU values can be selectively removed
- Metallic artifact
- High attenuation of endovascular coil mass or aneurysm clips can obscure
- Bone (skull base and posterior fossa)
- 3D reconstruction and surface shading software may be prone to artifacts
- Limited or judicious use in patients with iodine-based allergy and/or renal dysfunction
- May require contrast dose adjustment considerations for subsequent catheter angiography
- Typically, proceed to conventional angiography if NECT is consistent with SAH and CTA results are negative
- Beam hardening artifact
- Pitfalls
- Vessel loops and infundibula can mimic or be mistaken for aneurysms on CTA and MRA
- Vessel infundibulum
- <3-mm conical protrusion with vessel arising directly from apex (might not be definitive in some cases)
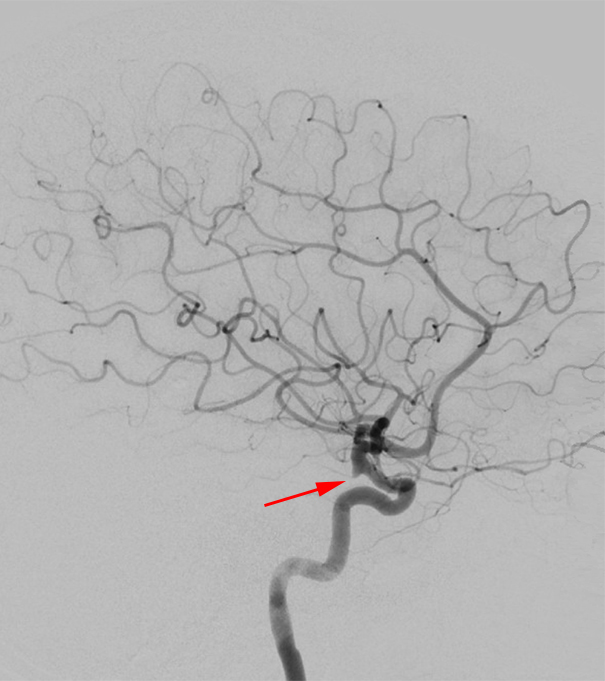
Figure 6: Lateral DSA image; the small infundibulum is clearly visible at the origin of the PComA from the distal ICA (arrow), but even without the visible presence of the PComA, small focal outpouching in this area implies an infundibulum rather than an aneurysm.
Magnetic Resonance Imaging
- Technique
- Multiple pulse sequences available to enhance various tissue properties as well as dynamic properties (flow, velocity)
- Ability to detect vascular flow
- High-velocity flow in arteries, veins, and aneurysms appears as a signal loss on MRI (“flow voids”)
- Occurs when protons in flowing blood exit the selected slice too quickly to acquire both the 90- and 180-degree pulses used to produce a spin echo
- Generally does not require intravenous contrast
- Spoiled gradient-recalled echo (SPGR) contrast-enhanced T1-weighted images (3D acquisition) may show best view of aneurysm
- Findings
- Very high sensitivity for detection of SAH
- FLAIR (87%) and SWI (88%)
- Combined FLAIR and SWI (~100%)
- Blood products (intra-axial or extra-axial) demonstrate five unreliable prototypical stages of evolution that are apparent on MRI:
- Hyperacute
- Intracellular oxyhemoglobin
- T1 isointense
- T2 isointense to hyperintense
- Acute (1–2 days)
- Intracellular deoxyhemoglobin
- T2-weighted signal intensity drops
- T1-weighted signal remains intermediate to dark
- Early subacute (2–7 days)
- Intracellular methemoglobin
- T1-weighted signal gradually increases, becoming hyperintense
- Late subacute (7 to 14–28 days)
- Extracellular methemoglobin; over the next few weeks, as cells break down, extracellular methemoglobin leads to an increase in the T2-weighted signal
- Chronic (>14–28 days)
- Hemosiderin can persist indefinitely, depending on the integrity of the blood–brain barrier
- Low T1-weighted and T2-weighted signals
- Superficial siderosis (hemosiderin coating the cortex)
- Uncommon
- Often due to chronic, repeated SAH
- Can cause atrophy and dysfunction of associated cortex
- Hyperacute
- Acute SAH will demonstrate patterns anatomically analogous (cisterns/sulci) to other axial imaging with variable signals depending on pulse sequence and predominate blood product composition
- T1-weighted imaging
- Isointense to mildly hyperintense (compared to cerebrospinal fluid [CSF])
- T2-weighted imaging
- Hyperintense
- Less sensitive given appearance similar to that of CSF
- FLAIR
- Hyperintense
- Highly sensitive within the first 5 days after SAH as CSF signal is nulled
- Gradient-recalled echo -T2*WI (GRE) and SWI
- Sequences show hemorrhage as black signal dropout as iron is prone to susceptibility artifacts
- T2*-weighted MRI is very useful in diagnosing previous SAH and may also reveal the location of a ruptured aneurysm
- Tends to exaggerate volume of blood with progressive stages ("blooming artifact”)
- DWI
- Generally negative, although blood products will behave variably with progressive stages
- May see parenchymal restricted diffusion (ischemic infarct) in territories of vessels affected by vasospasm
- T1-weighted imaging
- Aneurysm detection
- MRI is also highly sensitive for aneurysm detection
- Direct visualization of aneurysmal flow void
- Direct visualization of intra-aneurysmal contrast enhancement, particularly on SPGR postcontrast T1-weighted imaging
- Pulsation artifact
- Supportive finding seen in patent aneurysms, accentuated by contrast administration because of the increased intravascular signal
- Direct visualization of large and giant intracranial aneurysms and giant aneurysms
- Large and giant aneurysms are often partially thrombosed and can be confused with hematoma
- MRI is superior to CT and conventional angiography in characterizing large complex aneurysms
- Sequelae related to mass effect (ie, parenchymal edema on FLAIR imaging)
- MRI is also highly sensitive for aneurysm detection
- Very high sensitivity for detection of SAH
- Limitations
- Prone to multitude of artifacts that can be both helpful and difficult to interpret
- In general, less cost-effective and less available than CT
- CT is performed more easily in uncooperative, combative patients due to speed of acquisition
- MRI is contraindicated in the evaluation of postsurgical patients with a ferromagnetic aneurysm clip; for this reason, modern aneurysm clips are generally nonferromagnetic
- Pitfalls
- Flow-related enhancement and echo rephasing can cause increased signal intensity within normal vascular structures simulating aneurysms
- False diagnosis of basilar artery aneurysms is possible secondary to CSF pulsation artifact in the prepontine cistern
- Depending on the imaging parameters, turbulent and/or slow-flowing blood within a vessel or an aneurysm can have high or mixed signal intensity rather than a flow void
- Pneumatized bone (most notoriously, an aerated anterior clinoid) may be misinterpreted as an aneurysm flow void
Magnetic Resonance Angiography
- Technique
- 3D time-of-flight (TOF)
- Spatial resolution of modern 3D TOF MRA on the order of 1 mm3
- Axial source images
- Maximum intensity projection (MIP) images derived from source data
- 85% to 95% sensitive for aneurysms ≥2 to 3 mm
- Contrast-enhanced MRA
- Useful in surveillance of coil-treated aneurysms
- Metal suppression techniques can be employed, still being studied
- MRA should generally be considered complementary to conventional MRI
- MRA screening in patients with suspected familiar forms of cerebral aneurysms
- Dynamic contrast-enhanced MRA and DSA may prove useful as complex and slow flow patterns may generate artifacts limiting evaluation of vessel lumen on conventional MRI
- Vessel wall imaging
- Useful in evaluation or confirmation of dissection
- Time-of-flight (TOF) MRA can also demonstrate subacute intramural hematoma because of incomplete suppression completely of (stationary) tissues with intrinsic T1 shortening (phase-contrast MRA and contrast-enhanced MRA demonstrate only the vessel lumen)
- High resolution T1-weighted images with fat saturation can demonstrate crescent-shaped hyperintense false lumen around eccentric (narrowed) flow voids
- 3D time-of-flight (TOF)
- Pitfalls
- Intrinsic T1 shortening (T1 hyperintensity), such as in subacute hemorrhage or thrombosis, may simulate flow on TOF MRA
Digital Subtraction Angiography
- Preprocedural evaluation
- Preprocedural laboratory data
- Platelet count, prothrombin time, international normalized ratio, partial thromboplastin times to evaluate for a bleeding diathesis
- Blood urea nitrogen and creatinine levels to look for renal dysfunction
- Renal insufficiency predisposes to contrast-induced nephropathy
- Prehydration with sodium bicarbonate (130 mEq/L intravenous solution at 3.5 mL/kg bolus over 1 hour, then 1.2 mL/kg/hour during the procedure and for 6 hours after the procedure)
- Administration of N-acetylcysteine (600 mg orally at 24 and 12 hours before and after the procedure) may prevent contrast-induced nephropathy
- Noninvasive imaging and previous angiography should be reviewed for preoperative clinical assessment and treatment planning
- Physical examination
- Vital signs and cardiopulmonary status
- Complete neurological examination and documentation of any preprocedural deficits
- Extremity pulse evaluation, including bilateral dorsalis pedis and posterior tibial pulses for transfemoral approaches, and the Allen test for patients and procedures requiring radial artery access
- Contrast allergy
- True contrast allergy is rare
- If suspected, premedication with prednisone 50 mg orally at 24, 12, and 1 hour as well as diphenhydramine 50 mg orally or intravenously 1-hour preoperatively is a standard protocol to prevent an allergic reaction
- Informed consent before angiography should include an estimate of complication risk
- Neurological complications
- Cerebral ischemic events that occur as a result of thromboembolism, air emboli (more common)
- Disruption of atherosclerotic plaques and vessel wall injury such as perforation, dissection (less common)
- Overall rate of neurological complications is ~1%
- Transient/reversible complications occurring about 2× the rate of permanent complications
- Relative risk of complications increases with the following:
- Prolonged angiography procedure time
- Worse underlying primary disease process
- Underlying comorbidities (atherosclerotic carotid disease, recent cerebral ischemic event, advanced age, hypertension, diabetes, renal insufficiency)
- Neurological complications
- Preprocedural laboratory data
- Technique
- Conventional 4-vessel angiogram is considered the gold standard for aneurysm detection and characterization
- Biplane angiography
- Sterile prep
- Transfemoral approach is typical
- Modified Seldinger technique, short introducer sheath commonly used
- Longer sheath useful when high arterial tortuosity or high atherosclerotic burden might impair catheter navigation
- Catheter selection varies, typically 4F or 5F, advanced over hydrophilic wires
- Heparinized saline, double flushes
- Contrast, hand injected or power injected
- Bilateral internal and external carotid arteries
- Thorough evaluation of the anterior communicating artery complex
- May be necessary to perform an ICA cross-compression study
- Bilateral vertebral arteries, including bilateral posterior inferior cerebellar arteries
- Can be performed via a dominant vertebral artery
- Bilateral internal and external carotid arteries
- Multiple fluoroscopic projections
- Stereoscopy
- Views obtained in slightly different projections, viewed with prism glasses or by crossing eyes
- Can be valuable in complex cases, particularly in understanding venous anatomy and in AVM/AVF cases
- 3D rotational angiography reconstruction modeling
- Leads to more sensitive/specific aneurysm detection
- Provides invaluable anatomic information about aneurysm neck and geometry
- Catheter angiography effectively demonstrates anatomic detail such as aneurysm size, geometry, and neck-to-dome ratio
- Very high spatial resolution compared to current cross-sectional imaging paradigms
- Spatial resolution of flat-panel volume CT (rotational angiography using a C-Arm) ranges between 200 and 300 μm in high-resolution mode
- Spatial resolution of multislice CT scanners is up to 600μm
- Time-resolved, dynamic study
- Demonstrates collateral circulation and flow dynamics within the corresponding vascular territories
- Catheters can be exchanged over wires for triaxial microcatheter systems in endovascular therapies
- Findings
- Saccular aneurysm
- Saccular outpouching at arterial branch point
- Size and morphology
- Small (<0.3 cm) to giant (>2.5 cm)
- Vary in complexity from round to ovoid with daughter lobe(s)
- Narrow to wide-necked
- Branch vessel may be incorporated into aneurysm neck or dome
- Can lead to technical difficulties or preclude coil embolization
- Flow-diverting embolization devices becoming widely available for aneurysms with wide necks, unfavorable for coiling alone
- Anterior carotid circulation (85%-90%)
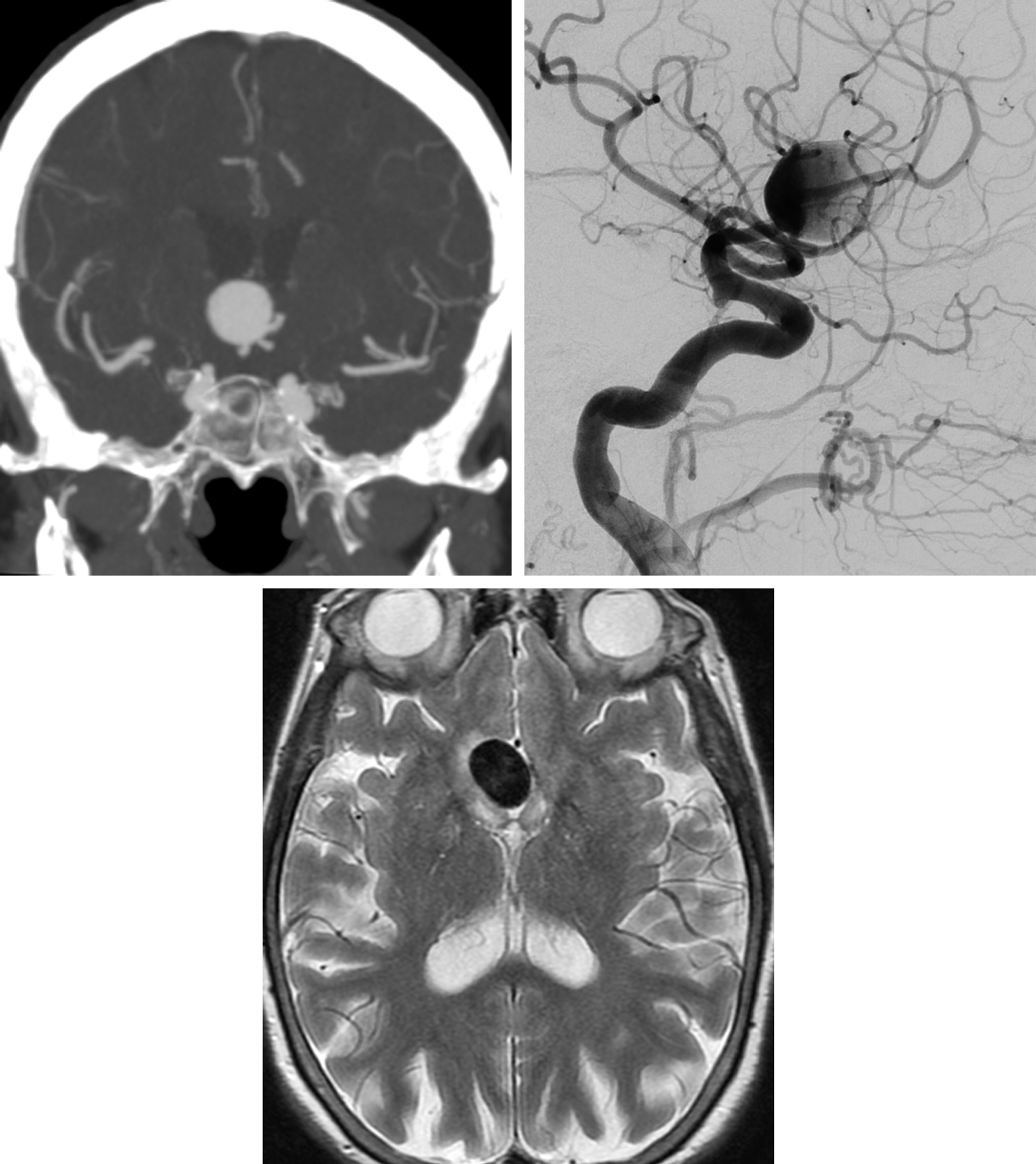
- Anterior communicating artery (30%)
- See Figure 6
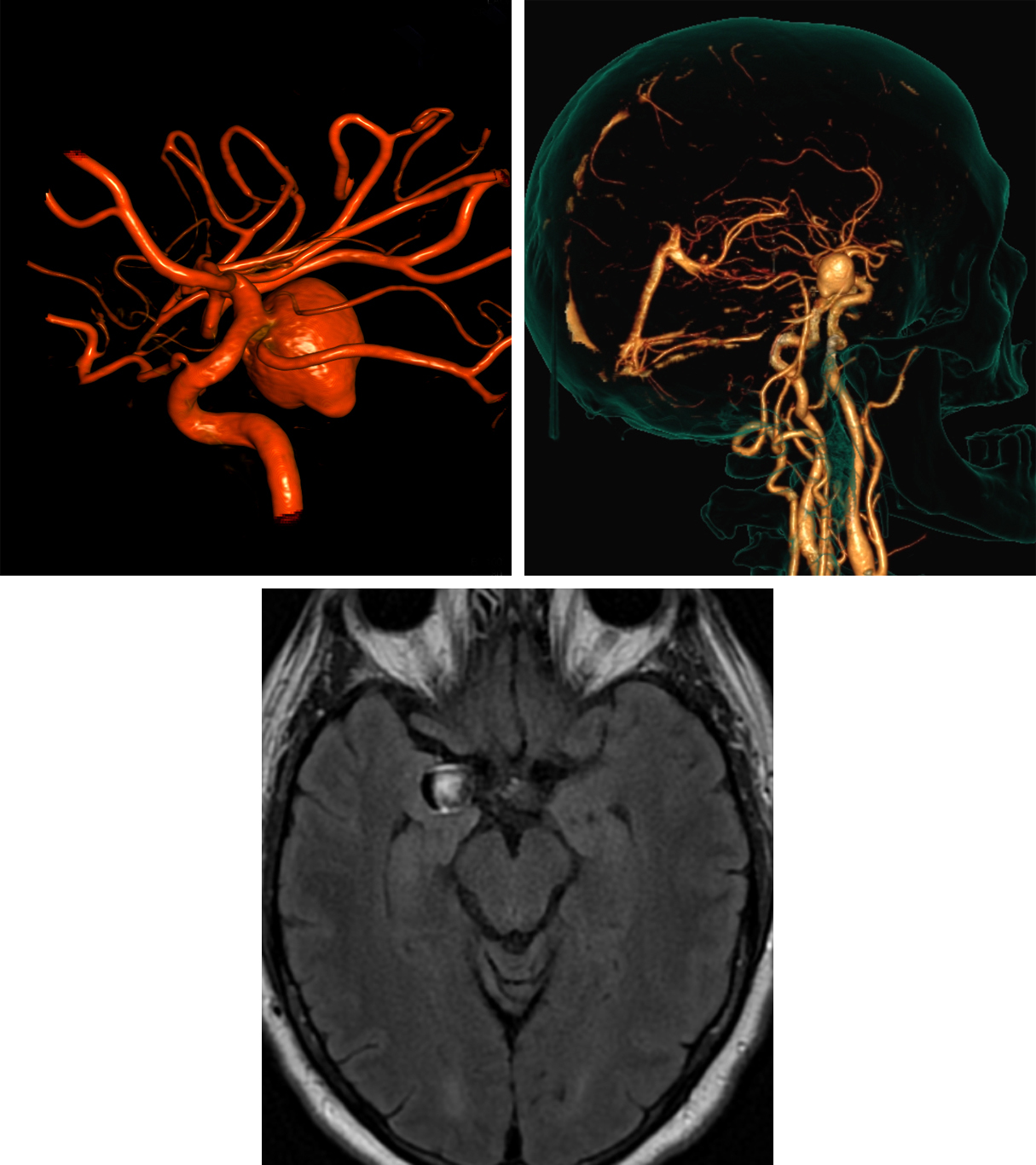
- Posterior communicating artery (25%)
- See Figure 7
- Middle cerebral artery bifurcation/trifurcation (20%)
- Anterior communicating artery (30%)
- Posterior vertebrobasilar circulation (10%-15%)
- Basilar artery trunk and bifurcation (basilar tip) (10%)
- Vertebral-posterior inferior cerebellar artery (3%)
- Rare locations (<<1%) include
- Pontine perforators, persistent trigeminal artery, fenestration aneurysms
- Multiple aneurysms (20%), can be familial cases
- Evaluate for segmental luminal narrowing of vasospasm in adjacent territory
- Active extravasation can be detected (rare)
-
For more information, please see the corresponding chapter in Radiopaedia
- Blister Aneurysm
- Smooth bleb-like outpouching from arterial side wall
- Typically small (< 6 mm length), wide neck
- Not necessarily associated with major arterial branch point
- Along the supraclinoid ICA near the carotid terminus
- MCA, ACA, ACoA, basilar artery are much more rare
- May be angiographically occult
- CTA may demonstrate subtle, asymmetric bulging of supraclinoid ICA
- Best evaluation with high-resolution DSA, multiple projections, and 3D angiography
-
For more information, please see the corresponding chapter in Radiopaedia
- Fusiform aneurysms resulting from advanced atherosclerotic disease
- Exaggerated dolichoectasia (“elongated and dilated”) with focal fusiform aneurysmal dilatations (basilar artery diameter of > 4.5mm)
- Tend to involve the vertebrobasilar arteries more often than carotid circulation
- Tend toward large or giant aneurysms (> 2.5 cm), indolent growth
- Propensity for cranial nerve compression syndromes
- Vertebrobasilar ectasia on trigeminal nerve
- Cavernous-supraclinoid carotid ectasia on optic nerve, chiasm
- Increased incidence of vertebrobasilar occlusions, brain stem ischemia/infarct
- Older patients
- Fusiform aneurysms resulting from vasculopathy
- Segmental ectasia due to inherited collagen-vascular diseases, viral, neurocutaneous syndrome, post-radiation
- Younger patients
- Dissecting Aneurysm and Pseudoaneurysms
- Luminal irregularity, abrupt narrowing/dilatation
- Pseudoaneurysmal saccular outpouching may be seen
- Locations
- Petrous/cavernous ICA, vertebral artery (more common)
- Relatively rare
- Potential etiologies of pseudoaneuryms
- Trauma (penetrating or blunt)
- Spontaneous dissection, underlying vasculopathy
- Infection, inflammation (mycotic aneurysm) usually occurring in unusual peripheral locations
- See Figure 8
- Associated with neoplasm
- Aneurysms associated with Vascular Malformations (discussed in other sections)
- Saccular aneurysm
Figure 7: AComA aneurysm. (Top Left) The large aneurysm arising from the anterior communicating artery demonstrates an ovoid appearance and homogeneous contrast filling on CT imaging during the arterial phase. (Top Right) After injection of the left ICA during DSA, the aneurysm also begins to fill during the arterial phase. (Bottom) T2-weighted MRI of the aneurysm shows the typical smooth black flow void that is characteristic of nonthrombosed aneurysms. A small amount of adjacent hyperintense edema may be reactive to the mass effect.
Figure 8: PComA aneurysm. (Top Left) This PComA aneurysm appears pedunculated but is clearly shown to incorporate the proximal (carotid) aspect of the PComA on this 3D reformat from DSA. A small bulbous outpouching from the aneurysm apex portends a higher risk for hemorrhage. (Top Right) CTA images can be reformatted to create images that may be helpful for anatomic localization for the neurosurgeon planning to clip an aneurysm. (Bottom Row) Axial FLAIR MRI demonstrates heterogeneity of this aneurysm due to flow-related signal changes.
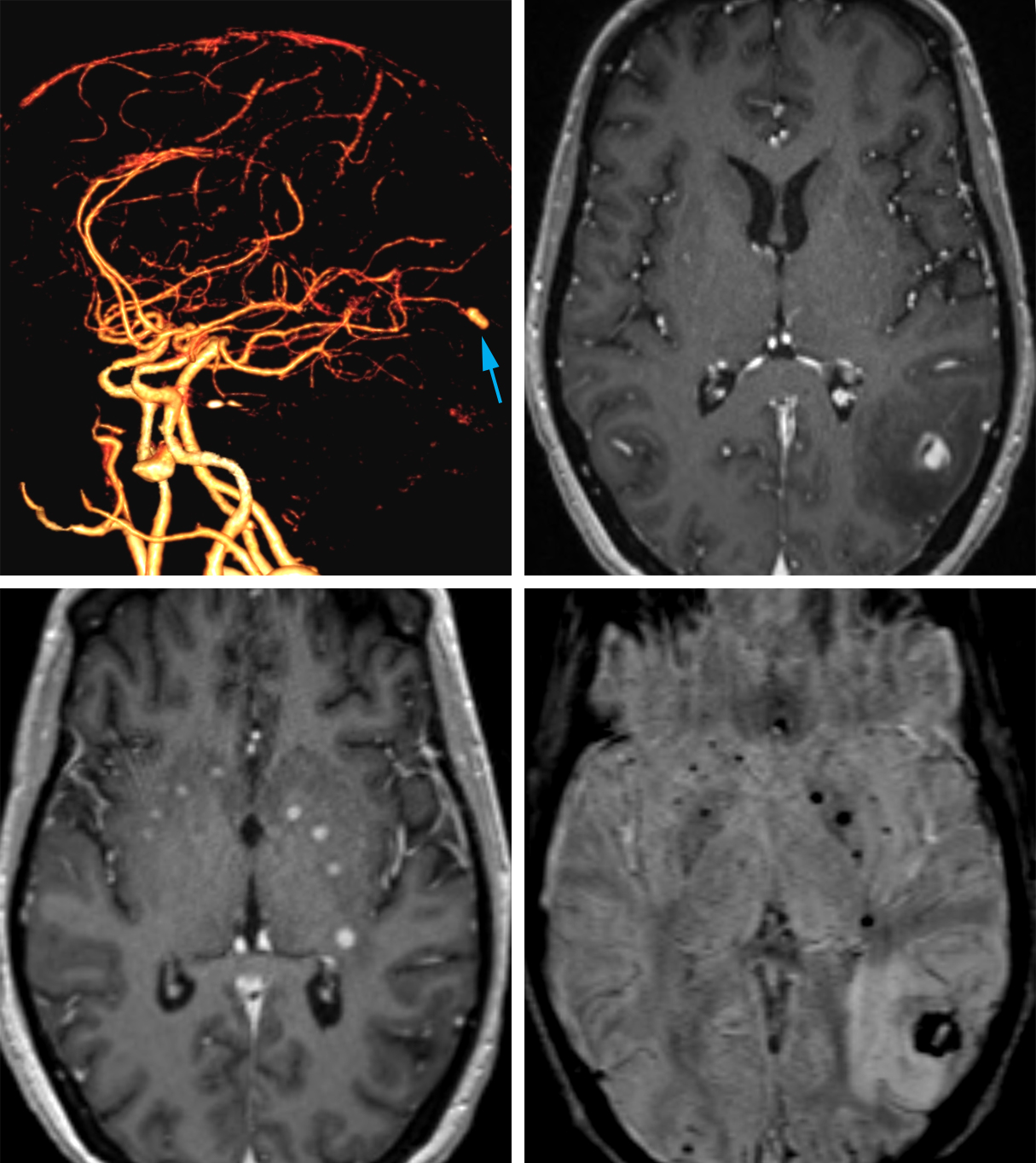
Figure 9: Mycotic aneurysm. (Top Left) The 3D reformatted CTA shows an aneurysm in an unusual location arising from the distal posterior cerebral artery (arrow). (Top Right) The contrast-enhanced T1-weighted MRI also demonstrates the location of this enhancing aneurysm in the lateral left occipital lobe with surrounding hypointense edema. (Bottom Left) More inferiorly, contrast-enhanced MRI also shows several small enhancing abscesses in and around the basal ganglia, often arising contemporaneously with mycotic aneurysms due to their shared etiology. (Bottom Right) These lesions are often associated with hemorrhage, as demonstrated by the low signal intensity areas on SWI MRI.
- Pitfalls
- DSA negative in 15% of aneurysmatic SAH, consider repeating DSA in 5 to 7 days
- Nonaneurysmal perimesencephalic SAH
- Up to 95% of cases will have normal cerebral angiogram, source of bleeding not identified
- Thought to be venous-origin bleed
- Evaluation and detection of saccular aneurysms depend on optimal projections
- Spontaneous partial or complete aneurysm thrombosis and presence of vasospasm
- Failure to evaluate the external carotid artery circulation may result in failure to demonstrate a dural AVF as cause of SAH
Summary and Recommendations
Imaging recommendations include initial evaluation of suspected SAH with NECT. If NECT results are positive for SAH, the study should be followed by multiplanar CTA, which gives important complementary anatomic detail to DSA. Proceed directly to DSA if NECT is consistent with SAH, but CTA fails to demonstrate the culprit aneurysm. Consider MRI with or without MRA in cases of large complex aneurysms with suspected mass effect and resultant cranial nerve dysfunction as well as in cases in which DSA and CTA fail to demonstrate an aneurysm. Lastly, rely on MRI with MRA in the surveillance of unruptured and treated aneurysms.
For more information, please see the corresponding chapter in Radiopaedia, and the Giant Cerebral Aneurysms chapter within the Brain Tumor Mimics subvolume of The Neurosurgical Atlas.
Contributor: Daniel Murph, MD
References
Farzad A, Radin B, Oh JS, et al. Emergency diagnosis of subarachnoid hemorrhage: an evidence-based debate. J Emerg Med 2013;44:1045–53. doi.org/10.1016/j.jemermed.2012.10.001
Froehler MT. Endovascular treatment of ruptured intracranial aneurysms. Curr Neurol Neurosci Rep 2013;13:326. doi.org/10.1007/s11910-012-0326-z
Imaizumi T, Chiba M, Honma T, et al. Detection of hemosiderin deposition by T2*-weighted mri after subarachnoid hemorrhage. Stroke 2003;34:1693–1698. doi.org/10.1161/01.STR.0000075771.88719.CE
Wallace RC, Karis JP, Partovi S, et al. Noninvasive imaging of treated cerebral aneurysms, part I: MR angiographic follow-up of coiled aneurysms. AJNR Am J Neuroradiol 28:1001–08. doi.org/10.3174/ajnr.A0662
Matsumoto K, Oshino S, Sasaki M, et al. Incidence of growth and rupture of unruptured intracranial aneurysms followed by serial MRA. Acta Neurochir (Wien) 2013;155:211–216. doi.org/10.1007/s00701-012-1566-z
Rabinstein AA. Subarachnoid hemorrhage. Neurology 2013;80:e56–e59. doi.org/10.1212/WNL.0b013e3182834b22
Vasan R, Patel J, Sweeney JM, et al. Pediatric intracranial aneurysms: current national trends in patient management and treatment. Childs Nerv Syst 2013;29:451–456. doi.org/10.1007/s00381-012-1945-z
Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol 2013;68:e15–e20. doi.org/10.1016/j.crad.2012.09.001
Cianfoni A, Pravatà E, De Blasi R, et al. Clinical presentation of cerebral aneurysms. Eur J Radiol 2013;82:1618–1622. doi.org/10.1016/j.ejrad.2012.11.019
Meling TR, Sorteberg A, Bakke, SJ, et al. Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg 2008;108:662–71. doi.org/10.3171/JNS/2008/108/4/0662
Verma RK, Kottke R, Andereggen L, et al. Detecting subarachnoid hemorrhage: comparison of combined FLAIR/SWI versus CT. Eur J Radiol 2013;82:1539–45. doi.org/10.1016/j.ejrad.2013.03.021
Please login to post a comment.