Low-Grade Diffuse Astrocytoma
Figure 1: T1-weighted postcontrast (left) and axial FLAIR (right) images demonstrate a fairly circumscribed infiltrative lesion involving the cortex and white matter. This low-grade tumor is associated with no appreciable enhancement.
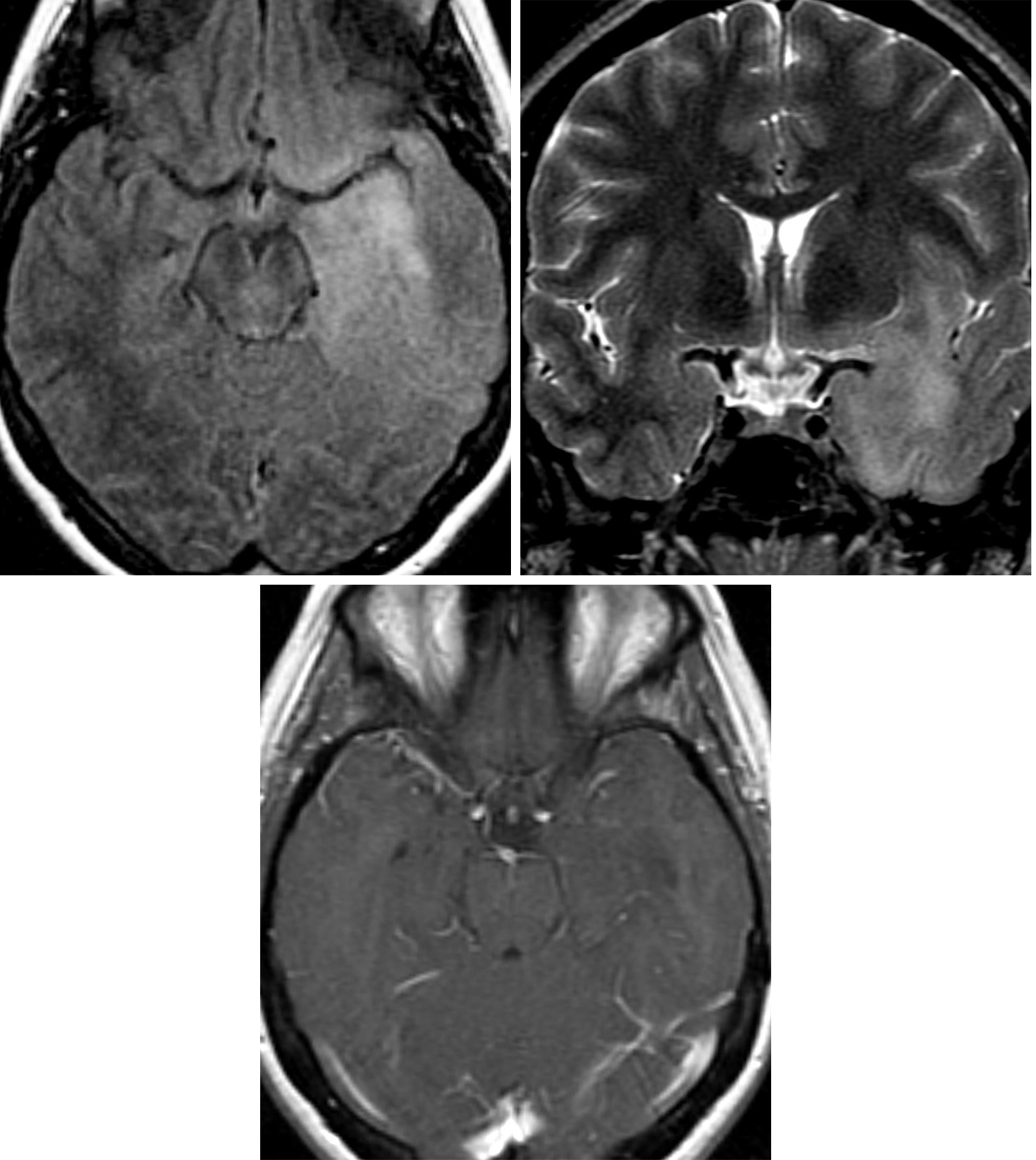
Figure 2: Axial FLAIR (top left) and coronal STIR (top right) images demonstrate a poorly defined infiltrative, hyperintense lesion involving the left temporal lobe, left insula, and inferior left frontal lobe. (Bottom) T1WI after contrast administration shows no contrast enhancement of this low-grade astrocytoma.
BASIC DESCRIPTION
- Primary tumor arising from well-differentiated astrocytes
PATHOLOGY
- WHO grade II
- Well differentiated, infiltrating, slow growing
- Malignant degeneration into anaplastic astrocytoma is common
CLINICAL FEATURES
- Commonly presents with seizures
- Average patient age, 34 years (20–45 years)
- Median survival, 6–10 years
- Survival greater in younger patients, gross-total resection, IDH1-, ARTX-, and MGMT-positive tumors
- Pontine tumors are associated with decreased survival
- Sometimes associated with Li-Fraumeni syndrome and Ollier disease
IMAGING
- General
- Infiltrating, focal, or diffuse white matter mass that distorts normal architecture
- Variable size; frontal lobe masses can be large at presentation
- Tumor commonly extends beyond region of signal abnormality
- Expansion of involved cortex
- Two-thirds are supratentorial; frontal lobe involvement is most common
- One-third are infratentorial; brainstem is most common, cerebellum is uncommonly involved
- Majority do not enhance
- Greater degree of enhancement suggests malignant degeneration
- ±Cysts, calcification (20%)
- CT
- Hypodense to isodense, poorly defined, homogenous mass
- ±Calcification
- Little to no enhancement on contrast-enhanced CT imaging
- MRI
- T1WI: homogenously hypointense
- T2WI: homogenously hyperintense
- FLAIR: homogenously hyperintense
- DWI: no restricted diffusion
- T1WI+C: little to no enhancement; greater degree of enhancement suggests higher WHO grade
- MR perfusion: low relative cerebral blood volume (rCBV) relative to anaplastic astrocytoma (AA) and glioblastoma multiforme (GBM); typically, the rCBV ratio to normal white matter is <1.8
- MRS: mildly elevated choline, mildly depressed N-acetyl aspartate (NAA) peaks and usually no appreciable lactate peak
IMAGING RECOMMENDATIONS
- MRI with contrast; consider MR perfusion for equivocal cases
For more information, please see the corresponding chapter in Radiopaedia.
Contributor: Rachel Seltman, MD
References
Appin CL, Brat DJ. Molecular genetics of gliomas. Cancer J 2014;20:66–72. doi.org/10.1097/PPO.0000000000000020.
Arevalo-Perez J, Peck KK, Young RJ. Dynamic contrast-enhanced perfusion MRI and diffusion-weighted imaging in grading of gliomas. J Neuroimaging 2015;25:792–798. doi.org/10.1111/jon.12239.
Law M, Oh S, Babb JS, et al. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology 2006;238:658–667. doi.org/10.1148/radiol.2382042180.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:547. doi.org/10.1007/s00401-007-0243-4.
Kleihues P, Cavenee WK. Pathology and genetics of tumours of the nervous system: diffuse astrocytoma. IARC Press, Lyon, France; 2000:22–26.
Ogura R, Tsukamoto Y, Natsumeda M, et al. Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology 2015;35:324–335. doi.org/10.1111/neup.12196.
Osborn AG, Salzman KL, Jhaveri MD. Diagnostic Imaging (3rd ed). Elsevier, Philadelphia, PA; 2016.
Wessels PH, Weber WE, Raven G, et al. Supratentorial grade II astrocytoma: biological features and clinical course. Lancet Neurol 2003;2:395–403. doi.org/10.1016/s1474-4422(03)00434-4.
Please login to post a comment.