Far Lateral Suboccipital (Transcondylar) Approach
This is a preview. Check to see if you have access to the full video. Check access
Nuances of Technique for Far Lateral Osteotomy
This is a preview. Check to see if you have access to the full video. Check access
Nuances of Technique for Far Lateral Approach and Resection of an Encasing Foramen Magnum Meningioma
General Considerations
The far lateral suboccipital and its variants, including the supra-, para- and trans-condylar approaches, have been developed to expose pathologies at the ventral and ventrolateral craniovertebral junction. This region includes the space anterior to the brainstem and the spinal cord, extending from the jugular bulb to the upper cervical spine.
This ventral region is difficult to reach microsurgically, and modifications of the far lateral suboccipital pathway require varying degrees of bony resection so that the pathology can be reached without retraction on the medulla oblongata and upper spinal cord. Anterior routes, such as the transnasal endoscopic and transoral trajectories, are reserved primarily for extradural lesions; the lateral corridors are preferred for intradural pathologies.
The far-lateral transcondylar approach entails a suboccipital craniotomy posterior to the sigmoid sinus and C1 hemilaminectomy followed by partial or complete resection of the ipsilateral occipital condyle. I rarely extend the condylectomy only to the point of adequate exposure because excessive condylar resection (>30%) increases the risk of occipitocervical instability and a need for occipitocervical fusion. This arthrodesis greatly limits the patient’s neck mobility. The far lateral approach without violation of the condyle is all that is needed in almost all cases.
In addition to the far-lateral transcondylar approach, extreme-lateral transcondylar and transjugular procedures have been described for extensive extradural lesions in this area. These approaches involve a complete condylectomy and partial mastoidectomy, and they provide wide exposure of the anterior craniovertebral junction. Yet they are associated with a high risk of surgical morbidity, require occipitocervical fusion, and have few if any indications. Therefore, I describe only the far-lateral approach here.
Indications for Far Lateral Approach
The most common indications for far lateral approach are:
- Anterior craniocervical junction intradural tumors, such as meningiomas or lower cranial nerve schwannomas.
- Vascular lesions, such as ventral aneurysms of the distal vertebral arteries and anterior lower brainstem cavernous malformations.
- Extradural clival lesions, such as chordomas, chondrosarcomas, metastases, or glomus jugulare tumors (the former two tumors are best approached through the transnasal endoscopic corridor).
Figure 1: This predominantly ventral foramen magnum meningioma was resected through the far lateral (conservative condylectomy) approach described below (upper images). The postoperative computed tomography scan (left lower image) demonstrates the minimal if any extent of condylectomy necessary. The vertebral artery was mobilized laterally as it entered the dura via the dural retention sutures. The intraoperative photo (right lower image) shows the generous exposure toward the anterior dura at the craniospinal junction and the final resection result. Extra-axial pathologies create ample amount of operative space upon their decompression.
Preoperative Considerations
Magnetic resonance (MR) imaging demonstrates the rostral-caudal extent of the tumor and guides the size of the suboccipital craniotomy. In addition, the ventral-lateral location of the lesion dictates the necessary extent of condylectomy. Brainstem and spinal cord edema and tumor calcification indicate pial invasion and correlate with development of postoperative neurologic decline since some degree of pial manipulation is necessary to mobilize the tumor. In these cases, a subtotal resection, while leaving a thin sheet of the tumor on the pia and surrounding vessels to protect the perforators, is recommended. Encasement of vessels and infiltration of the tumor into the jugular foramen (encasement of cranial nerves) are other indications for subtotal resection.
Imaging studies should include computed tomography (CT) or catheter angiography to evaluate the relationship of the adjacent vasculature (vertebral artery, intradural versus extradural origin of the posterior inferior cerebellar artery) to the pathology and the laterality of vertebral artery’s dominance. This information affects the safety of sacrificing the ipsilateral vertebral artery proximal to the posterior inferior cerebellar artery. Highly calcified tumors can present daunting challenges in their resection and require decompressive surgery because they encase neurovascular structures.
Figure 2: This young patient’s calcified meningioma was observed for years until gait disturbance interfered with his daily life (upper images). An initial attempt at its resection at an outside institution was futile. Another attempt at resection was undertaken. The highly calcified nature of the tumor complicated tumor decompression that was accomplished via drilling the core of the tumor. During mobilization of the capsule away from the brainstem, I inadvertently injured the encased vertebral artery. My aggressive approach led to postoperative brainstem strokes and the unfortunate demise of this patient (lower images). This case taught me the importance of decompression rather than aggressive resection in highly calcified foramen magnum tumors.
The patient’s preoperative evaluation should include a thorough neurologic and otolaryngologic exam with particular attention to the lower cranial nerves. Detailed evaluations of the swallowing and vocal cord functions are necessary. If there is concern regarding preoperative lower cranial nerve palsy, the patient should be informed of the need for a tracheostomy and/or gastrostomy postoperatively. Malnourished patients affected by preoperative swallowing dysfunction should be treated first through nutritional consultation to minimize the risk of postoperative wound breakdown or overall recovery,
After these evaluations, the surgeon plans the expected extent of condylar resection based on a detailed study of the MR and CT images. As I mentioned above, the far lateral approach without violation of the condyle is all that is needed in almost all cases. In very rare cases, I believe the removal of less than 30% of the condyle may be needed to safely resect isolated ventral lesions. Almost always, the pathologic lesion itself has widened the required operative corridor toward the anterior brainstem and eliminates the need for more extensive condylar resection.
I do not advocate medial mobilization of the vertebral artery (extreme far lateral approach) to further the condylectomy. In fact, this maneuver is unnecessary and increases the risk of injury to the artery and hypoglossal nerve and portends biomechanical instability.
Intraoperative monitoring of brainstem auditory evoked responses (BAERs) and somatosensory evoked potentials (SSEPs) as well as electromyographic monitoring of cranial nerves VII, X, XI, and XII are recommended.
Operative Anatomy
The following images from Dr. Albert Rhoton’s work further illustrate the relevant surgical anatomy described later in this chapter. A detailed understanding of the complex anatomy of the craniocervical junction is important for complication avoidance.
Click here to view the interactive module and related content for this image.
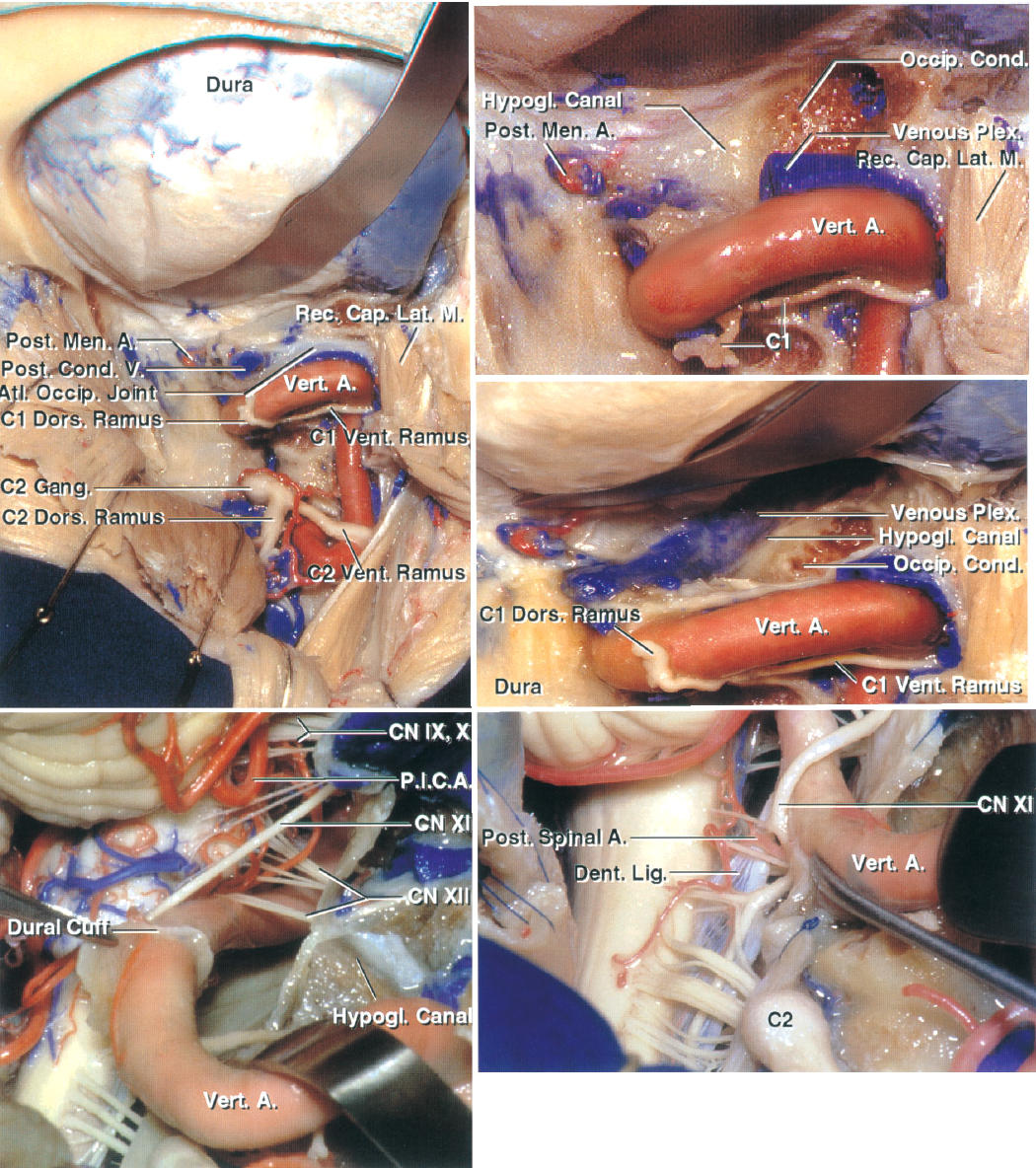
Figure 3: The hashed area in the left upper image defines the maximal required extent of condylectomy for purely ventral craniocervical lesions. Early identification of the vertebral artery on the lateral arch of C1 (sulcus arteriosus) is the first step in this approach. Note the location of the posterior condylar vein as a landmark to assist with the extent of condylectomy (right upper image). The vein is along the lateral aspect of the condyle. Once I reach the vein, I drill only the outer shell of the condyle. The lower images demonstrate other relevant anatomy of the region including cranial nerve XII. A., artery; Atl., atlanto-; Car., carotid; CN, cranial nerve; Cond., condyle; Dent., dentate; For., foramen; Hypogl., hypoglossal; Jug., jugular; Lig., ligament; N., nerve; Occip., occipital; P.I.C.A., posterior inferior cerebellar artery; Post., posterior; Proc., process; Stylomast., stylomastoid; Trans., transverse; Vert., vertebral. (Images courtesy of A. L. Rhoton, Jr.)
Click here to view the interactive module and related content for this image.
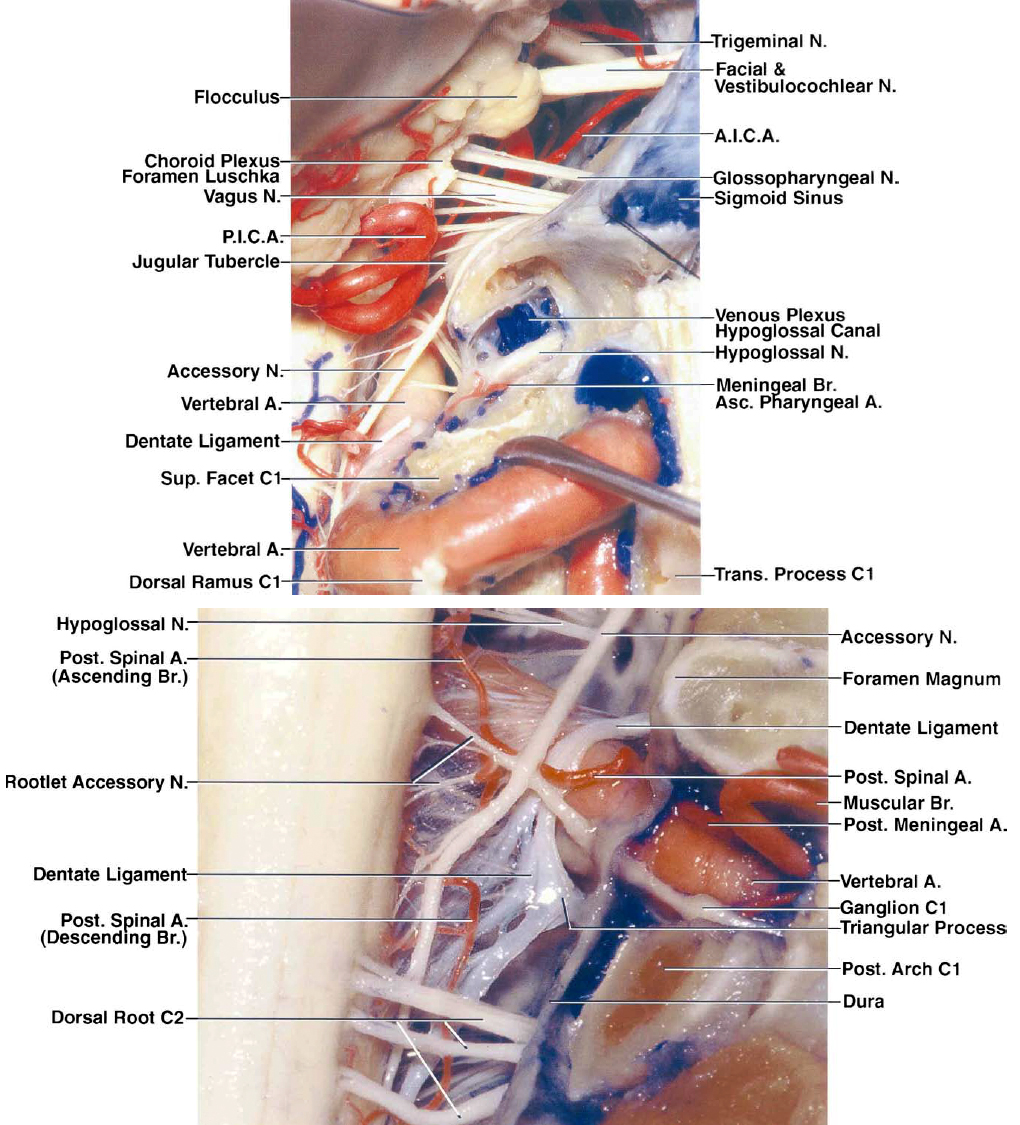
Figure 4: These images further detail the extra- and intradural anatomy at the craniocervical junction. The location of the C2 ganglion is evident; its retraction or transection leads to dense numbness in its distribution. Note the location of cranial nerve XII (left lower image). Dissection of dentate ligaments expands the operative corridor. A., artery; Atl., atlanto-; Cap., capitis; CN, cranial nerve; Cond., condyle/condylar; Dent., dentate; Dors., dorsal; Gang., ganglion; Hypogl., hypoglossal; Lat., lateralis; Lig., ligament; M., muscle; Men., meningeal; Occip., occipital; P.I.C.A., posterior inferior cerebellar artery; Plex., plexus; Post., posterior; Rec., rectus; Vent., ventral; Vert., vertebral. (Images courtesy of A. L. Rhoton, Jr.)
Figure 5: These images further demonstrate the complex neurovascular relationships of the craniocervical region. The upper image indicates the relevant anatomy of the right hypoglossal nerve after a complete condylectomy. Note the muscular branches of the vertebral artery and their location (lower image); their avulsion may lead to torrential bleeding, but this bleeding should not be confused with bleeding from the parent vessel. The C1 rootlets and 1 or 2 upper spinal rootlets of the accessory nerve may be sacrificed to expose large tumors. A., artery; A.I.C.A, anterior inferior cerebellar artery; Asc., ascending; Br., branch; N., nerve; P.I.C.A., posterior inferior cerebellar artery; Post., posterior; Sup., superior; Trans., transverse. (Images courtesy of A. L. Rhoton, Jr.)
Figure 6: Simulation of the operative perspective and related anatomy after a partial condylectomy has been executed. The dura has been removed. A reasonable pathway toward the ventral dura near the craniocervical junction is available. Tumors in this region displace the neurovascular structures and expand the operative corridor. (Image courtesy of A. L. Rhoton, Jr.)
FAR LATERAL OSTEOTOMY
Patient positioning and the degree of head rotation can substantially affect the operative corridor during this approach.
Figure 7: (Top) The patient is placed in the park-bench or lateral position. An axillary roll supports the contralateral axilla. The ipsilateral shoulder is gently pulled anteriorly and inferiorly and secured with tape. All pressure points are well padded, and the patient is secured and taped to the table at the hip and lower extremities to allow for safe tilting of the table during the later stages of the operation. The tape should be away from the head of the fibula to prevent common peroneal nerve neuropathy. (Bottom) Degree of head rotation. The mastoid bone is the highest point on the head.
I avoid significant neck rotation, but slightly turn the patient’s head toward the floor to maintain the relative normal anatomic course of the vertebral artery at the craniocervical junction for surgical orientation.
A number of different incisions, including the “lazy S” and “reserve U” incisions, have been described for transcondylar route. I use the traditional hockey-stick incision because it minimizes dissection through the neck muscles and mobilizes the myocutaneous flap laterally out of my working zone.
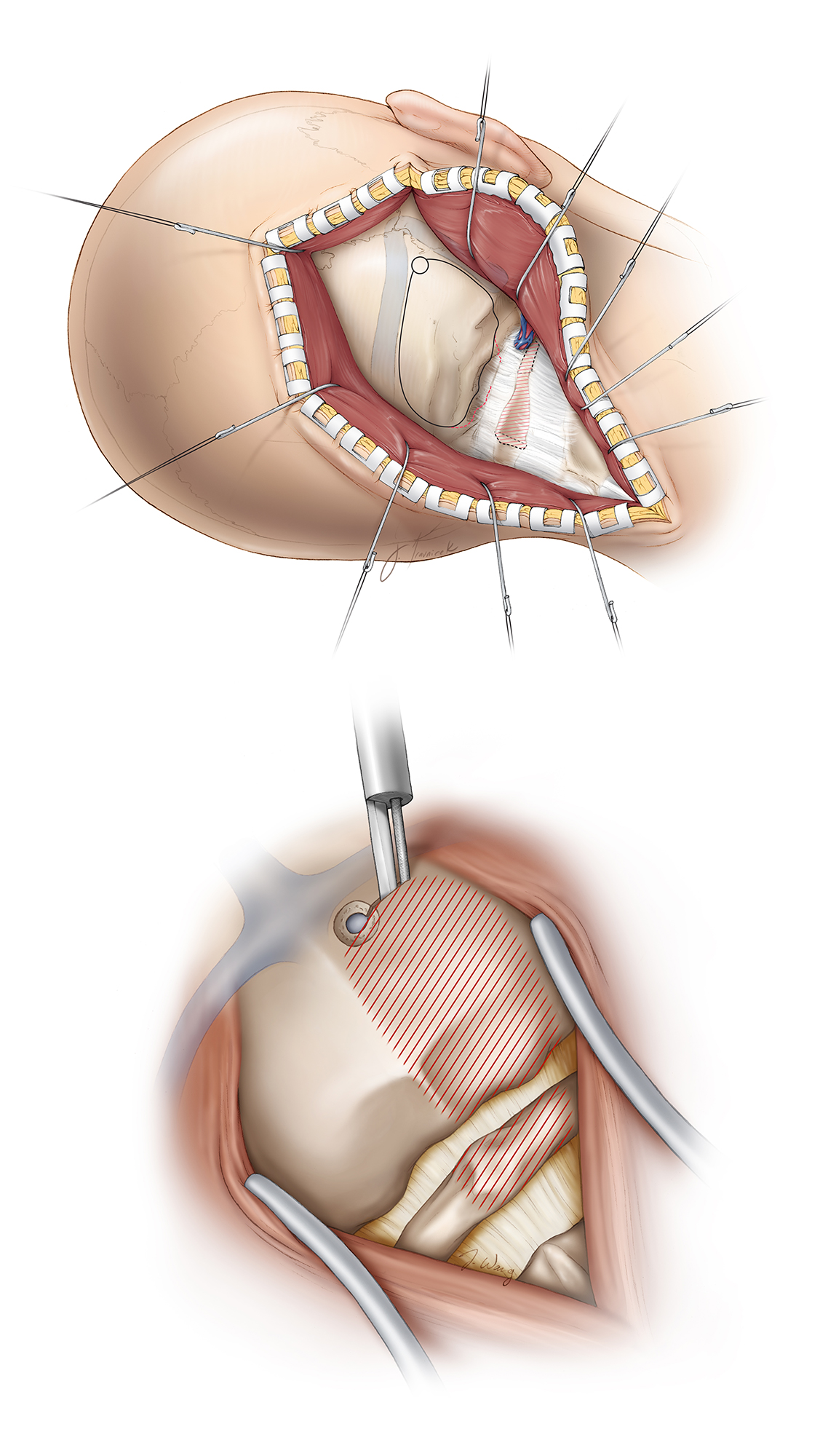
Figure 8: I use a “hockey-stick” incision beginning about 3 cm above the mastoid tip and extending superiorly, then curving medially just below the superior nuchal line to the midline, where it extends inferiorly to the level of the C2–C3 spinous processes. Next, a deeper cut is made below and parallel to the superior nuchal line to leave a 1-cm cuff of muscle that will facilitate closure. The midline nuchal ligament is identified and the paraspinal muscles are divided within this avascular plane. The muscles overlying the occipital bone and C1/C2 laminae are mobilized in the subperiosteal plane, and the myocutaneous flap is reflected inferolaterally.
Once the myocutaneous flap is mobilized laterally, great care must be taken to identify the vertebral artery in its sulcus over the lateral arch of C1 and avoid its injury. Once subperiosteal dissection reaches the lateral half of the C1 lamina, electrocautery is abandoned in favor of meticulous sharp and blunt dissection to identify the artery and its encasing venous plexus.
Figure 9: As the vertebral artery emerges from the transverse foramen of C1, it turns medially and rests on a bony depression on the superior surface of C1 called the sulcus arteriosus. The artery proceeds in a horizontal course until it pierces the atlanto-occipital membrane just medial to the edge of the C1 lateral mass, where it becomes intradural. Doppler ultrasonography may be used during repeat operations to identify the artery within the scar. Moreover, in older patients, highly ectatic vertebral arteries may reside within the paraspinal muscles, outside the sulcus arteriosus, and careful muscle dissection is warranted. The right vertebral artery encased by its venous plexus is shown.
The myocutaneous flap is then secured with fishhooks, and I use a fine curette to dissect the atlanto-occipital ligament away from the edge of the foramen magnum and the hemilamina of C1. The vertebral artery is then followed into the transverse foramen. The posterior arch of the atlas and its lateral mass are also exposed in a subperiosteal fashion. The ligaments around the artery are microsurgically dissected so that the artery can be mobilized laterally during reflection of the dura.
Venous bleeding from the periarterial plexus can be controlled using low-amplitude bipolar coagulation or thrombin-soaked Gelfoam packing and tamponade.
Figure 10: Next, the lateral suboccipital craniectomy or craniotomy and C1 hemilaminectomy are performed. A craniotome may be used, but it is often difficult to maneuver the footplate through the thickened bone at the lip of the foramen magnum. For this reason, I perform a craniectomy. The drill is also used to shell out the ipsilateral half of the C1 lamina; a Kerrison rongeur is then utilized to complete the hemilaminectomy.
Figure 11: Once the bone flap is removed and the hemilaminectomy completed, I excise additional bone using a combination of the drill and Kerrison rongeurs. The bony exposure typically extends from near the midline to the medial aspect of the occipital condyle, depending on the exact position of the tumor. Lateral suboccipital craniectomy exposes the posterior aspect of the sigmoid sinus leading to the jugular bulb. The hemilaminectomy extends to the level of the C1 lateral mass.
Figure 12: I resect the lateral suboccipital bone and rarely if ever drill through the occipital condyle to accentuate the exposure (upper two images). There is often an emissary vein near the lateral aspect of the condyle that is a useful landmark. This intradiploic vein can cause a fair amount of bleeding, but the bleeding is easily controlled with bone wax or Gelfoam packing. I continue to remove the rim of foramen magnum and follow the posterior condylar vein while drilling the posterior cortex of the condyle. This maneuver is adequate for providing a reasonable operative corridor toward the ventral brainstem (lower image, arrow points at the extent of condylectomy that ends just supralateral to the dural entry point of the vertebral artery). The superior articular process of the C1 lateral mass is resected just lateral to the dural entry point of the vertebral artery.
If a more extensive condylectomy is absolutely necessary, the surgeon must prevent injury to the hypoglossal nerve as it courses through the superior aspect of the anterior third of the condyle.
The superior articular process of the C1 lateral mass may also be partially removed. Resection of half or more of the occipital condyle results in biomechanical instability and necessitates occipitocervical arthrodesis.
INTRADURAL PROCEDURE
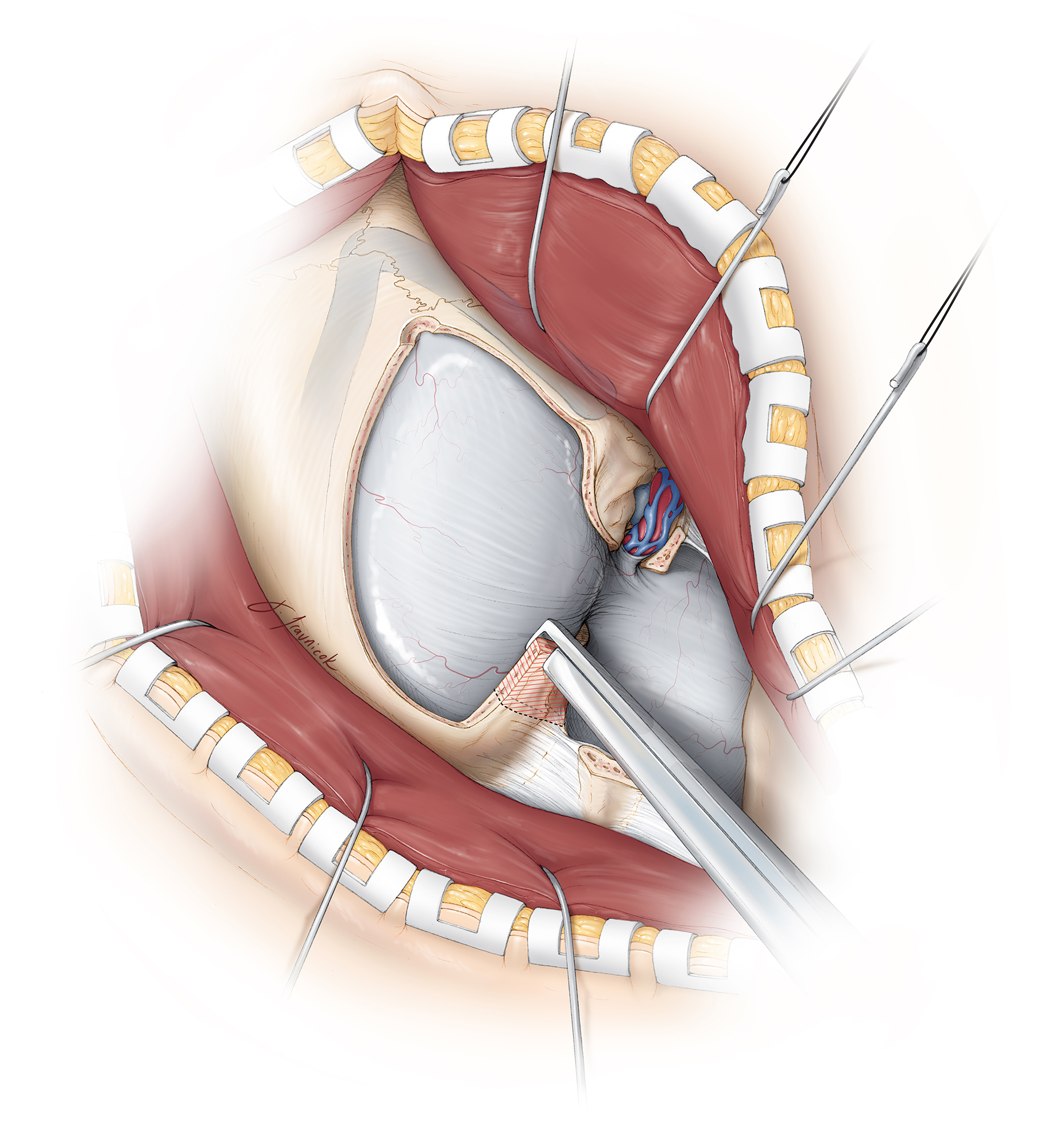
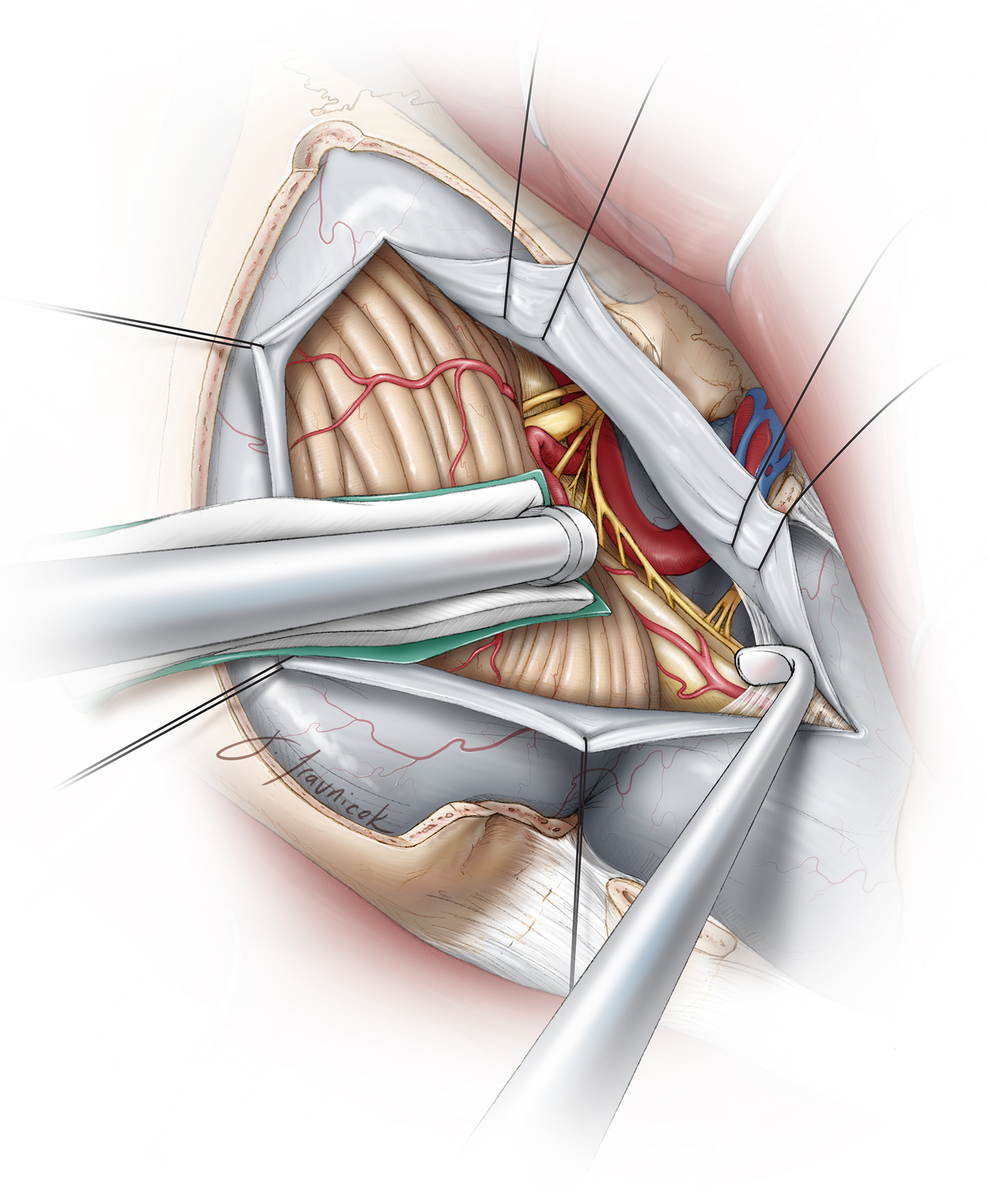
Figure 13: The dura is incised in a curvilinear fashion. Care is taken during dural incision to avoid intradural neurovascular structures, particularly the intradural vertebral artery and its branches, as well as a potentially posteriorly displaced spinal accessory nerve. The tumor usually displaces the proximal intradural segment of the vertebral artery laterally.
Figure 14: Intraoperative images demonstrate the generous exposure of a right anterolateral foramen magnum meningioma (upper image) through the far lateral route. The C1 rootlets and 1 or 2 upper spinal rootlets of the accessory nerve (upper image–at the tip of the scissors) may be sacrificed to expose this large tumor. The accessory nerve is then mobilized laterally along the posterior capsule of the tumor. The final resection result is also shown (lower image). As you can see in the lower image, this minimalistic approach to condylectomy allows adequate access to the ventral craniospinal junction.
The dural edges are tacked up using 4-0 sutures and kept moist throughout the operation to avoid their desiccation by the intense heat of the microscope, facilitating watertight closure. The tack-up stiches are placed along the bony edges (the most anterior aspect of the exposed dura) to maximize mobilization of the dural flaps away from the operative trajectory. This maneuver mobilizes the vertebral artery as it enters the dura, laterally and out of the working trajectory of the surgeon.
The cisterna magna may be opened to drain cerebrospinal fluid, relax the cerebellum, and improve exposure without the use of fixed retraction. At this point, the relevant pathology is addressed.
Closure
Once the pathology is surgically treated, absolute hemostasis is achieved in the operative site and the surgeon’s attention turns to closure. The subarachnoid space is copiously irrigated, and the dura is closed primarily, if possible, or via a dural graft. Every effort must be made to achieve a watertight closure. I do not routinely replace the bone flap or complete a cranioplasty because the bony defect is small. There is no concern regarding delayed instability in the craniospinal junction after the above approach. The soft tissues are then closed in the anatomic layers.
Pearls and Pitfalls
- Condylectomy rarely if ever is needed. A partial condylectomy (<30%) suffices to achieve adequate exposure of the anterior aspect of the brainstem in very rare cases.
- Preoperative studies should evaluate vertebral dominance and exclude the possibility of the ipsilateral vertebral artery ending only in the posterior inferior cerebellar artery. Any aberrant location of the dolichoectatic vertebral artery extending outside its sulcus arteriosus and hiding within the posterior muscle layers should be noted. In this variation, the artery is vulnerable to injury during dissection.
- Muscular branches of the vertebral artery tether the artery to the posterior soft tissues; these branches should be carefully coagulated and transected. Their avulsion injury may lead to parent vessel occlusion.
- To protect the vertebral artery, I avoid monopolar electrocautery on the lateral half of the C1 lamina as the posterior wall of the sulcus arteriosus may be incompetent. Aggressive coagulation of the vertebral venous plexus should be avoided to prevent arterial injury. Thrombin-soaked Gelfoam may be used to achieve hemostasis.
For additional illustrations of the far lateral approach to the foramen magnum, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of infratemporal fossa approaches, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of transjugular craniotomy, please refer to the Jackler Atlas by clicking on the image below:
References
Al-Mefty O. Operative Atlas of Meningiomas. Lippincott-Raven, Philadelphia, PA; 1998.
Cusimano M, Faress A, Chang Y, et al. Foramen magnum meningiomas. In DeMonte F, McDermott M, Al-Mefty O, eds, Al-Mefty’s Meningiomas, 2nd ed. Thieme Medical Publishers, New York, NY; 2011:297–306.
Sen C, Shrivastava R, Anwar S, et al. Lateral transcondylar approach for tumors at the anterior aspect of the craniovertebral junction. Neurosurgery 2010;66:A104–A112. doi.org/10.1227/01.NEU.0000365930.95389.60.
Sen C, Sekhar LN. Extreme lateral transcondylar and transjugular approaches. In Sekhar LN, Janecka IP, eds, Surgery of Cranial Base Tumors. Raven Press, New York, NY; 1993:389–411.
Please login to post a comment.