Ventricular Tumors
This chapter includes a discussion of important diagnostic, clinical, intraoperative, and postoperative tenets for the most common lesions within the ventricular cavities. These lesions are listed in their relative order of incidence in Table 1. Please also refer to Table 1 of the Principles of Intraventricular Surgery chapter for relevant radiologic features of these tumors.
| Colloid cyst |
| Craniopharyngioma |
|
Fibrillary astrocytoma |
|
Meningioma |
|
Ependymoma |
|
Chordoid glioma |
|
Germ cell tumors |
Colloid Cyst
Colloid cysts represent 0.5% to 1% of intracranial lesions. These cysts predominantly originate from the roof of the third ventricle at the foramen of Monro and uniquely contain a viscous gelatinous material. The cysts can reach a substantial size and be hemorrhagic (intracystic hemorrhage). They also can exceedingly rarely precipitate sudden occlusion of the foramen of Monroe and resultant life-threatening acute hydrocephalus.
The method of sudden obstruction of the foramen of Monro can be related to acute intracystic hemorrhage, postlumbar puncture transposition of the tumor mass, or shunt malfunction.
Radiographic differential diagnosis for a colloid cyst includes third ventricular cystic tumors, posterior circulation aneurysms, neurocysticercosis, and vertebrobasilar dolichoectasia. These lesions do not generally increase significantly in size overtime; however, intracystic hemorrhage is not rare.
Management of a colloid cyst is dependent upon the patient’s clinical state and the cyst’s size. Asymptomatic lesions can be managed conservatively with periodic radiographic evaluation. Unlike larger cysts, small cysts (<10 mm) have a low risk of becoming symptomatic.
If the patient’s hydrocephalus is symptomatic, there is an indication for surgical resection, either via endoscopic or microsurgical techniques. Please see the chapters on transcallosal and transcortical resection of colloid cysts.
Figure 1: Colloid cysts do not usually enhance but demonstrate T1 isointensity. On T2 weighted images, they show hyperintensity compared to the white matter with a circumferential rim of hypointensity.
Craniopharyngioma
Craniopharyngiomas represent 1.2% to 4.6% of intracranial tumors and are generally regarded as benign slow-growing lesions but their location and propensity to encase neurovascular structures leads them to recur and demonstrate a more aggressive clinical course.
These tumors have a bimodal peak incidence in patients who are either 5-10 or 45-60 years old. In children, they are the most common type of non-neuroepithelial intracranial lesion. The origin of these tumors is from the ectodermally-derived Rathke’s pouch within the parasellar area.
The clinical presentation of a craniopharyngioma often derives from its mass effect on structures of the optic pathway, anterior pituitary, or ventricular structures, resulting in obstructive hydrocephalus. The treatment of choice for these lesions is microsurgical resection.
The operative approach for these lesions involves the endoscopic transnasal transsphenoidal, extended pterional transsylvian, or rarely interhemispheric transcallosal route. Unless a complete radical resection is performed, the craniopharyngioma can be expected to recur. Therefore, radiotherapy is an important part of the care of residual tumors.
Figure 2: Craniopharyngiomas demonstrate heterogeneous cystic and solid features on MR imaging (top left and right). They show T1 hyperintensity within their cystic components and intense heterogeneous contrast enhancement of the nodule (lower left). CT scan (lower right) demonstrates punctate calcification.
Pilocytic Astrocytoma
Pilocytic astrocytoma tumors are World Health Organization (WHO) grade I, benign, slow-growing, tumors. These tumors may originate from the midbrain, thalamus, or hypothalamus, and expand into the third ventricle.
They are most commonly within the cerebellum and extend into the fourth ventricle, but they may also originate along the fourth ventricular roof and spread progressively into the lateral recesses and the brachium pontis. A dominant cystic component is usually present.
The surgical approach to a pilocytic astrocytoma of the fourth ventricle resembles the approaches for ependymomas and medulloblastomas, optimally through the telovelar approach. A combined telovelar and supracerebellar exposure may be necessary for large tumors extending superiorly to the level of the tectum with invasion of the superior medullary velum.
Visualization of the superior margin of the tumor may require retraction of the vermal uvula and nodulus. This region may be the site of tumor origin. If the telovelar approach limits verification of total resection, the surgeon may also consider using the supracerebellar approach.
The tumor’s cyst is first drained, and the tumor nodule is then dissected using standard microsurgical techniques. Safe subtotal removal is the goal. Most residual tumors of this type have a low risk of progression.
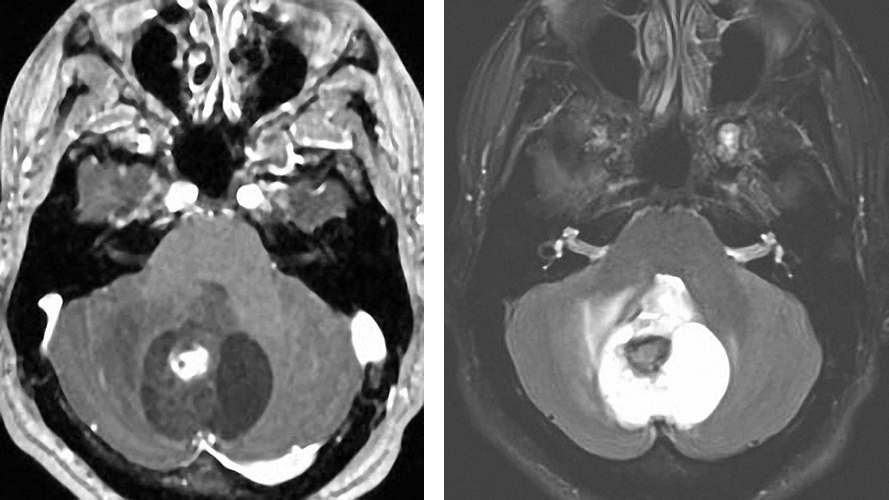
Figure 3: A vermian pilocytic astrocytoma with extension and compression of the fourth ventricle is demonstrated. The tumor is cystic with an enhancing nodule. The wall is not enhancing and does not require removal.
Pineocytoma
These WHO grade I lesions are responsible for 1% of intracranial tumors that originate from the pineal parenchyma and occupy the pineal region. They are generally benign, very slow-growing lesions and associated with exceedingly rare occurrence of malignant progression.
The clinical presentation of these tumors results from their mass effect on the tectum causing Parinaud’s syndrome, cranial nerve palsies, endocrine abnormalities, altered mental status, or obstructive hydrocephalus. Microsurgical or endoscopic fenestration via the supracerebellar corridor is a reasonable option.
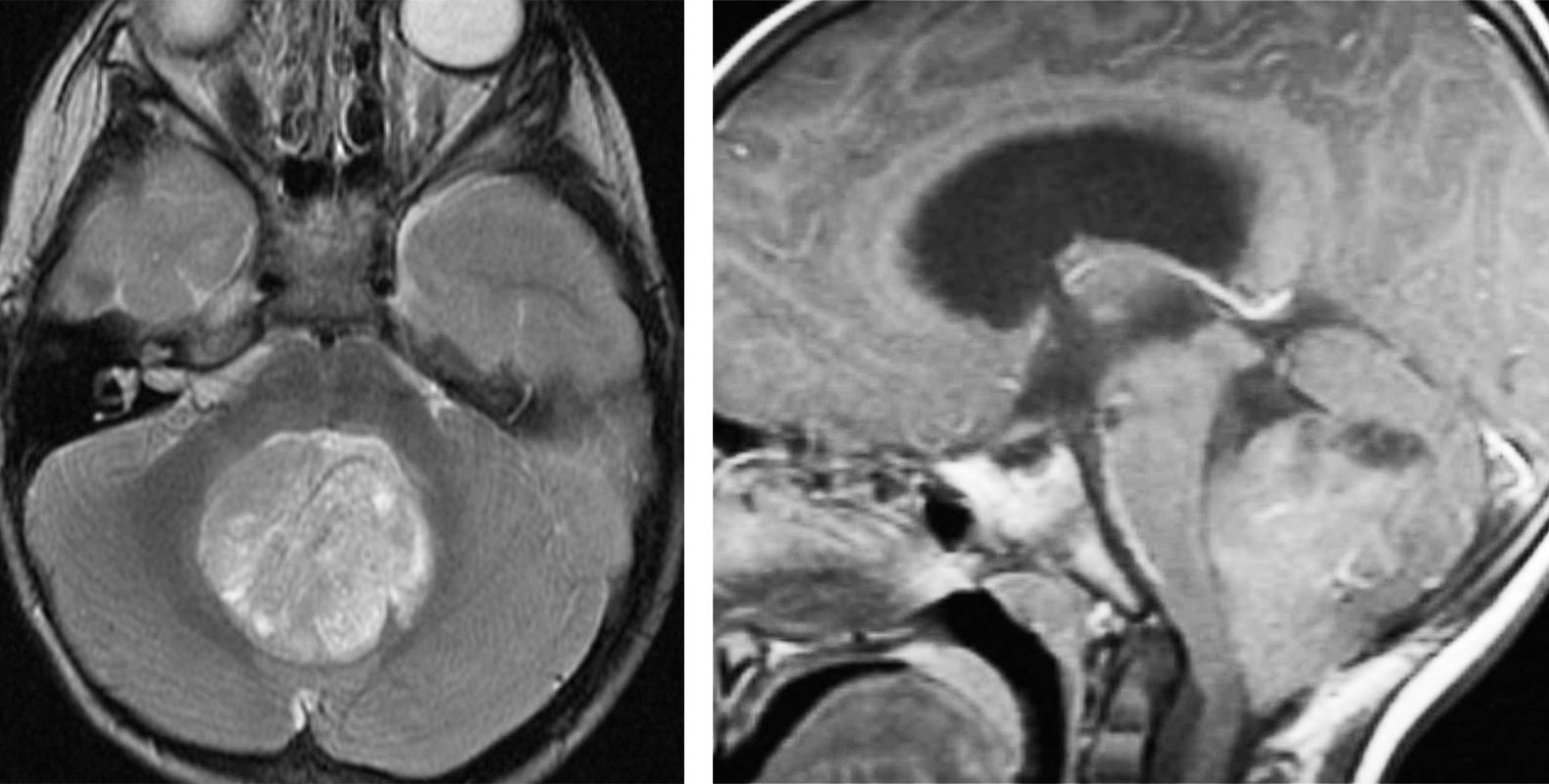
Figure 4: A pineocytoma demonstrates T2 hyperintensity and contrast enhancement at its periphery. Pineocytomas typically grow slowly and occasionally require operative intervention.
Cavernous Malformations
The incidence of these lesions is rare and approaches 0.1%-0.5%. When involving the 4th ventricle, they primarily arise from the brainstem. Paraventricular cavernous malformations are often asymptomatic, but can generate obstructive hydrocephalus because of their mass effect. Acute presentation may occur with their hemorrhagic transformation within the ventricular system, precipitating neurologic decline.
The exposure of the fourth ventricular cavernous malformations involves the telovelar approach. Electrophysiologic mapping of the rhomboid fossa may be necessary for localizing and protecting the facial colliculus before an incision along the floor is made.
After the safe entry zones are identified, a small incision along the floor allows hematoma drainage. Coagulation of the internal caverns facilitates debulking of the lesion. The resection proceeds in a circumferential pattern with segmental removal of the malformation. The surgeon should complete the resection with scrupulous coagulation of the feeding vessels to eliminate microfoci and facilitate hemostasis.
For more information, please refer to the chapters related to cavernous malformations including the one titled Evaluation of Cavernous Malformation.
Medulloblastoma
These tumors account for the majority of fourth ventricular tumors in the pediatric population. Medulloblastomas can occupy the entire fourth ventricle, generating mass effect on the brainstem and cerebellum. Unlike ependymomas, these lesions do not invade the ventricular floor, but instead originate from the superior medullary velum. Insertion points for medulloblastomas are at the brachium pontis and lateral recesses.
The optimal surgical approach for these lesions is the telovelar approach. Initial debulking of the tumor mass should evacuate the superior rhomboid fossa to bring the cerebral aqueduct into view. The resection then continues inferiorly. It is imperative to avoid perforating the floor of the 4th ventricle. Lateral extension of medulloblastomas is common.
Figure 5: Medulloblastomas are hypointense to grey matter on T1 and often heterogeneously enhance. On T2 sequences, they also appear heterogeneous due to calcification, necrosis and cyst formation.
High-Grade Glioma
High-grade gliomas (WHO grades III and IV) are commonly derived from the corpus callosum, thalamus, or septum pellucidum. These lesions demonstrate a partiality for the anterior horn of the lateral ventricle.
The clinical presentation of these highly malignant lesions results from compression or infiltration of the surrounding parenchyma. A decision on management for these lesions is multifaceted because of their overall poor prognosis. Particular factors of interest include the patient’s age, neurologic status, neuropsychological function, and lesional characteristics such as size, depth of invasion, and location.
Extensive forniceal involvement is associated with poor neurologic status and the need for a biopsy rather than surgical resection.
Figure 6: High-grade gliomas heterogeneously enhance with irregular borders and nodules. They also appear hyperintense on T2 sequences. Tumors of the septum pellucidum can mimic neurocytomas.
Subependymoma
Subependymomas account for 0.2%-0.7% of intracranial tumors and are generally considered benign, slow-growing lesions adherent to the ventricular wall. These tumors do not invade the peritumoral parenchyma. The most common location for them is the fourth ventricle, followed by the lateral ventricle.
The radiologic differential diagnoses for this lesion include ependymomas, oligodendrogliomas, and choroid plexus papillomas. The clinical presentation of subependymomas often involves obstructive hydrocephalus, depending on the location and size of the lesion.
The management of incidentally identified lateral ventricular subependymomas is controversial, and an acceptable approach is routine follow-up evaluation with imaging to identify the evolution of the mass. I do not pursue surgical resection in patients with asymptomatic subependymomas.
Figure 7: Subependymomas do not enhance significantly and are hyperintense to adjacent white and grey matter on T2 weighted images. On T1 images, they are iso- or hypointense. The lesion on the above images is located at the floor of the fourth ventricle.
Fibrillary Astrocytoma
These tumors are WHO grade I astrocytomas that, when intraventricular, predominately occur in the lateral ventricle. Fibrillary astrocyomas demonstrate a scattered infiltrative growth pattern, but are considered benign slow-growing lesions with low malignant potential. These tumors emerge from the ventricular wall.
The clinical presentation of these lesions typically involves obstructive hydrocephalus. They have a propensity to become hemorrhagic and can cause intraventricular hemorrhage.
Similar to subependymomas and central neurocytomas, the most appropriate management of incidentally identified low-grade gliomas is debated. Asymptomatic patients with small incidentally identified lesions can undergo surveillance imaging.
Meningioma
Meningiomas account for 1-5% of all ventricular tumors. When they arise from the lateral ventricle, their origin involves the stroma of the choroid plexus and tela choroidea. They frequently harbor calcifications and cystic degeneration.
Their high vascularity can offer an operative challenge, with most of their anterograde blood supply arising from the choroidal arteries and retrograde venous backflow joining the deep ventricular veins. Control over the feeding vessels early in surgery may be impossible. This limitation may lead to excessive bleeding during piecemeal tumor removal. Stepwise hemostasis is necessary and operative planning should consider the need for exposure of the tumor’s vascular pedicle early in surgery.
Clinical presentation of these lesions commonly involves obstructive hydrocephalus because of the tumor-derived mass effect. Management of these lesions includes radical microsurgical resection. For tumors identified incidentally, routine clinical and radiographic analysis is an appropriate course, and progression is an indication for surgical resection.
Figure 8: Meningiomas demonstrate intense homogenous enhancement with very well-demarcated borders and dural tails. They are typically hyperintense on T2 weighted images. The atrium is their most common intraventricular location.
Central Neurocytoma
Central neurocytomas account for 0.1-0.5% of intracranial tumors and are classified as WHO grade II lesions. Most of these lesions arise from the septum pellucidum, fill the lateral ventricles with a propensity for the left frontal horn.
Although primarily benign, the identification of malignant features with postresection remnant progression is an unfavorable prognostic sign. If malignant progression is identified, there is a role for adjuvant therapies (chemotherapy and radiation); both are associated with a reported reduction in tumor volume with undetermined prognostic efficacy.
These tumors are frequently highly vascular and their stereotactic biopsy may lead to life-threatening intraventricular hemorrhage. Attention to immaculate hemostasis during their circumdissection is advised.
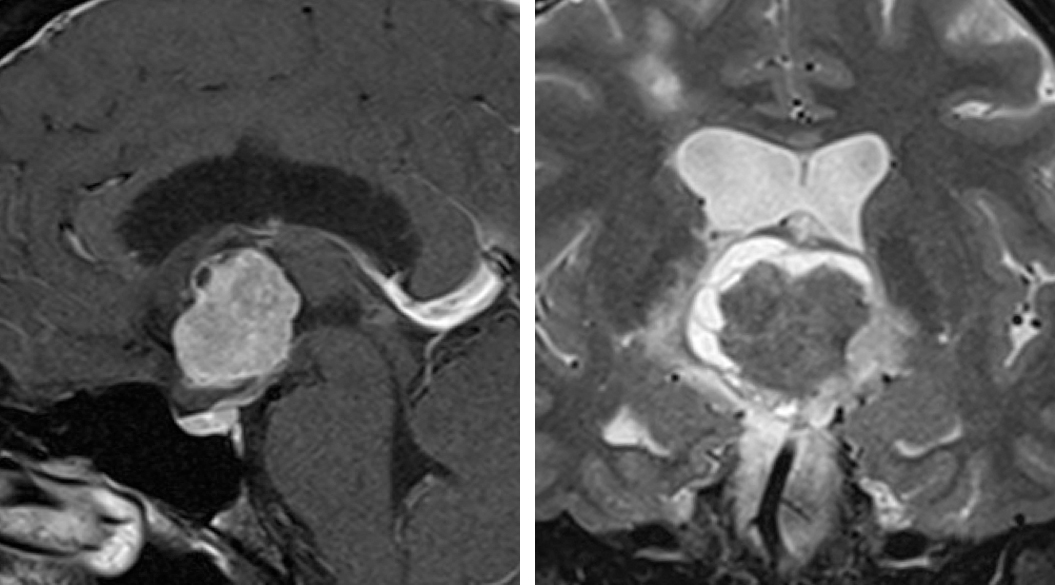
Figure 9: Neurocytomas demonstrate heterogeneous enhancement patterns and appear iso- to hyperintesne on T2 weighted images. They originate from the septum pellucidum.
Ependymoma
Ependymomas are predominantly identified in the pediatric population and account for 2-9% of intracranial tumors. However, in adults, ependymomas are the most common fourth ventricular tumor originating from the ventricular floor.
These tumors often possess a vascular pedicle at the floor of the ventricle as their only site of association with the parenchyma. They are generally considered benign, slow-growing lesions.
Resection of fourth ventricular ependymomas is tedious. The tumor is first debulked until its apex is separated from the inferior surface of the ventricular roof. This maneuver will relieve obstructed cerebrospinal fluid pathways. This superior section of the tumor often crowds the ventricle, but is not adherent to the superior rhomboid fossa.
Subsequently, the inferior aspect of the tumor can be divided from the neural parenchyma at the obex and resected. This technique facilitates gradual resection of large lesions and eventual isolation of the lesion to the inferior rhomboid fossa. Ultrasonic aspiration assists in resection of the tumor piecemeal. A well-defined plane does not separate the tumor from the dorsal brainstem; the ultrasonic aspirator shaves off enough tumor while a small sheet is left over the brainstem. Aggressive resection of the infiltrating tumor is not advised.
Lateral extension of fourth ventricular ependymomas into the lateral recesses or cerebellopontine angle is not a contraindication to gross total resection. Elevation of the tonsils allows exposure of these spaces for tumor mobilization.
Surgical resection correlates with a favorable prognosis and is the treatment of choice for ependymomas. If histopathology of the ependymoma reveals malignant features, adjuvant radiotherapy is necessary.
Figure 10: Ependymomas are iso- to hypointense on T1 images. They are T2 hyperintense and show heterogeneous enhancement patterns.
Epidermoid Cyst
Epidermoid cysts are rare ventricular lesions; they occur mainly within the fourth ventricle and can extend into the third ventricle and lateral recesses. The cyst can be multiloculated because of its septations. The outer surface of the cyst may incorporate pial vessels.
Gentle microdissection is used to sharply divide the cyst from the brainstem and cerebellum while preserving the invested pial vessels on the tumor capsule. Next, the cyst in the fourth ventricle can be debulked using an ultrasonic aspirator or a suction device. The dense arachnoid membranes/septations compose the walls of the loculations within the cyst. These septations can be released but should be left behind if adherent to neurovascular structures.
Following removal of the mass, perimedullary spaces and lateral recesses should be irrigated with saline solution to sweep away any residual microscopic tumor foci, which could serve as a source for recurrence.
Figure 11: Epidermoid cysts are isointense to CSF on T2 weighted images. Diffusion weighted imaging is beneficial for their differentiation from arachnoid cysts based on their increased signal due to a combination of true restricted diffusion and T2 shine through.
Hemangioblastoma
These lesions most commonly present within the fourth ventricle and are distinctly different from ependymomas or medulloblastomas. Their solid counterparts can be highly vascularized, perfused principally by the distal branches of the posterior inferior cerebellar arteries, end arteries along the brainstem and cerebellum, and choroidal vessels.
Hemangioblastomas also involve venous vasculature. This level of arterial and venous involvement necessitates a preoperative angiogram for operative planning, identification of major vasculature encompassed within the tumor, and an opportunity for embolization.
The operative technique for removal of hemangioblastomas is unique in that these lesions cannot be debulked. The parenchyma of hemangioblastomas resembles those of AVMs made of thin-walled honeycomb channels that are not readily amenable to bipolar coagulation. Before removal, the key step involves identification and sacrifice of the terminal, and not en passage major arterial feeders, to the lesion.
Upon restriction of arterial blood flow to tumor segments, the tumor may decrease in size and facilitate exploration of its capsule, including the fourth ventricle. The devascularized portions of the lesion may then be segmentally dissected away from the surrounding neurovascular tissues; this step is repeated in a circumferential pattern.
It is paramount to maintain patency of the major draining veins until the lesion is completely separated from the cerebellum and brainstem.
There can be significant pial supply, which can maintain perfusion to segments of the lesion. Therefore, complete dissection of the lesion from the brainstem and cerebellum must be completed to ensure complete removal of the perfusing arterial supply. After this is achieved, the major draining veins can be coagulated to facilitate removal of the lesion. For more details, refer to the chapter on hemangioblastomas.
Pineoblastoma
These infrequent tumors are ranked WHO grade IV, although they account for approximately 40% of primary pineal tumors. They are identified primarily in the pediatric population and have no sex predilection. An occurrence of ventriculoperitoneal shunt-mediated dissemination has been reported.
Options for therapy include microsurgical resection or diagnostic biopsy followed by adjuvant therapy. Safe gross total resection is advised. The tumor can infiltrate the tectal region, but usually can be dissected effectively. The entire neuroaxis should be imaged to rule out metastatic lesions.
Figure 12: Pineoblastomas typically are T2 isointense and heterogeneously enhance upon administration of contrast.
Chordoid Glioma
These rare glial tumors are WHO grade II and most commonly originate within the third ventricle. The treatment of choice is gross total resection. If complete resection is not achieved, the tumor will recur. Adjuvant therapies are not a proven option for the treatment of choroidal gliomas due to their poor efficacy in clinical trials.
This tumor tends to adhere to the floor of the ventricle; aggressive resection at this site is not advised. The most common approach to this type of tumor is a transcallosal expanded transforaminal route.
Choroid Plexus Papilloma
These lesions account for 0.5-0.6% of intracranial tumors and are considered WHO grade I lesions. Choroid plexus papillomas are most often found in the pediatric population.
Management should involve surgical resection as the treatment of choice. Choroid plexus papillomas are highly vascular. Intraoperative pearls include early impediment of proximal choroidal feeding arteries, facilitating devascularization of the tumor mass. Debulking with an ultrasonic aspirator follows this step.
Following removal of the tumor mass, the remaining nonpathologic appearing choroid plexus should be resected to decrease the risk of recurrence.
Figure 14: Choroid plexus papillomas intensely enhance with administration of contrast and have very irregular borders. They are also hyperintense on T2 weighted images.
Germ Cell Tumor
These tumors represent 0.4-3.4% of intracranial tumors, with germinomas making up the majority (65%). Less common types include teratomas (18%−20%) and mixed germ cell tumors (25%). Most cases are found in the pediatric population (10−21 years old). Germ cell tumors are rare in patients older than 30 years.
The pineal region and third ventricle are primary sites of involvement. Therefore, the clinical presentation results from mass effect, generating obstructive hydrocephalus, cranial nerve palsies, optic pathway disruption, cerebellar dysfunction, or endocrine abnormalities.
Options for therapy include microsurgical resection, endoscopic removal, diagnostic biopsy, and adjuvant therapy. For more details, please refer to the chapter on pineal region tumors. Preoperative characterization of these tumors through cerebrospinal fluid studies and endoscopic biopsy is recommended.
Subependymal Giant Cell Astrocytoma (SEGA)
The SEGAs are WHO grade I lesions that originate from the lateral ventricular wall. They demonstrate a special predilection for patients suffering from tuberous sclerosis and their presence is a poor prognostic sign. In contrast, in the general population, they are considered benign slow-growing lesions with a low malignant potential.
The clinical presentation of SEGAs typically involves obstructive hydrocephalus or intraventricular hemorrhage.
Similar to other low-grade gliomas, the best strategy for an incidentally identified SEGA is debated. In asymptomatic patients with small incidental lesions, follow-up radiographic imaging is most reasonable.
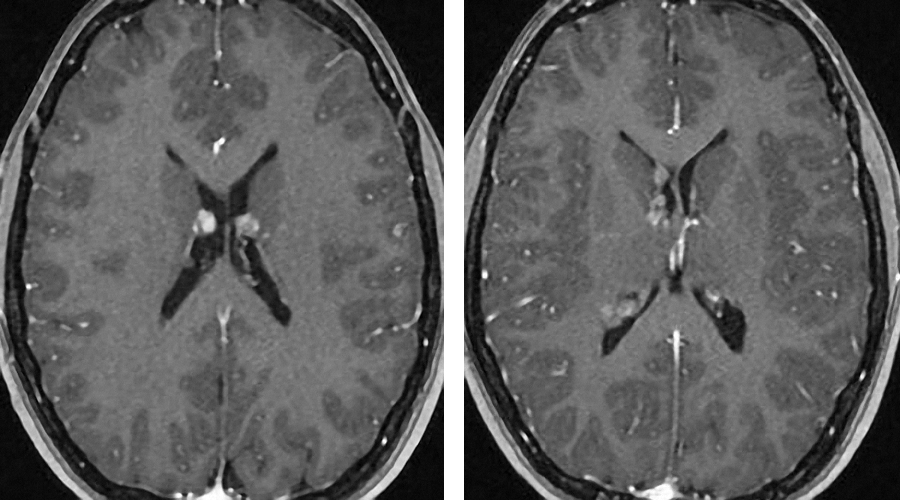
Figure 15: Multiple subependymal nodules of SEGA are present. They have a special predilection for the region of the foramen of Monro.
Contributor: Benjamin K. Hendricks, MD
References
Bertalanffy H, Krayenbühl N, Wess C, Bozinov. Ventricular tumors (Chapter 138), in Winn HR (ed): Youmans Neurological Surgery, Vol 1, 6th Ed, Philadelphia: Elsevier Saunders, 2011, 1534-1568.
Please login to post a comment.