Peri-insular Hemispherotomy
This is a preview. Check to see if you have access to the full video. Check access
Techniques of Modified Peri-Insular Hemispherotomy for Hemimegalencephaly
Indications for surgical consideration in patients with drug-resistant epilepsy include a defined epileptogenic focus and a low likelihood of new neurologic deficit after surgery. In adults, the most common etiology is temporal lobe epilepsy, that is, mesial temporal sclerosis. The most common epileptogenic etiologies in pediatric surgical candidates are low-grade tumors and malformations of cortical development.
Surgical options for pediatric patients who have marked dysfunction of a single epileptogenic hemisphere have evolved over time. Complications resulting from highly resective operations such as anatomic hemispherectomy, including superficial siderosis and secondary hydrocephalus, have led to the development of less resective and more disconnective functional hemispherectomy. Functional hemispherectomy has recently given rise to hemispherotomy, the least resective operation primarily aimed at disconnecting the abnormal hemisphere. Hemispherotomy is effective in reducing or eliminating seizure frequency and likely decreases the risk of postoperative complications when compared with its predecessors.
Hemispherotomy is a technically challenging operation that requires a thorough understanding of three-dimensional cerebral anatomy to ensure adequate hemispheric disconnection without placing the deep structures at risk or leaving certain hemispheric connections intact that could lead to residual postoperative seizures.
In this chapter, I will discuss the relevant operative nuances for a modified form of peri-insular hemispherotomy. Through hemispherotomy, experienced surgeons can effectively treat patients with unilateral epileptogenic hemisphere dysfunction while limiting potential complications. First I will briefly review the historical evolution of hemispherectomy and hemispherotomy techniques.
Historical Perspectives and Evolution of Techniques
Hemispherotomy evolved from hemispherectomy. Walter Dandy first performed the latter operation for treatment of malignant gliomas in 1928, and hemispherectomy for treatment of epilepsy was first reported in 1938. Superficial siderosis was later recognized as a potential long-term complication. Up to 33% of patients developed this condition at a median time of 8 years after the procedure. Superficial siderosis occurs as a consequence of chronic granular ependymitis associated with multiple bleeding areas on the membrane that replaces the resected hemisphere in continuity with the ventricular system, leading to neurologic decline, hydrocephalus, and sometimes death.
To avoid this complication, less resective modifications of the operation were implemented, such as functional hemispherectomy. In this procedure, the frontal and occipital poles are disconnected but not removed, with the goal of achieving similar seizure control as with hemispherectomy while preventing the delayed complication of superficial siderosis. This operation has continued to be modified in an attempt to minimize cerebral resection and also to decrease intraoperative blood loss while maintaining its effectiveness, leading to hemispherotomy techniques.
| Complications | |
| Hemispherectomy | Blood loss |
| Superficial siderosis | |
| Hydrocephalus with higher rate of postoperative shunting | |
| Functional hemispherectomy | Decreased average blood loss when compared to above |
| Hydrocephalus with lower rate of postoperative shunting | |
| Higher rate of recurrent seizures and need for reoperation | |
| Hemispherotomy | Hydrocephalus |
| Residual seizures | |
| Postoperative hemorrhage | |
| Lowest blood loss | |
| Shortest ICU stay | |
| Lower overall complications rate |
Preoperative Evaluation and Surgical Indications
Severe unihemispheric dysfunction causing medically refractory epilepsy in the pediatric population is frequently selected for surgical treatment through hemispherotomy. Some of the most common indications are multilobar cortical dysplasia, hemimegalencephaly, polymicrogyria/polypachygyria, posttraumatic epilepsy, Rasmussen encephalitis, perinatal stroke, and Sturge-Weber syndrome. The goal of surgery in these patients is to interrupt the spreading pathways of the epileptic discharge in order to isolate the epileptogenic zone.
Children suffering from refractory seizures who have received two unsuccessful but appropriate trials of anticonvulsant medications should be referred for surgical evaluation. Pediatric patients suffering from epilepsy may develop developmental regression or arrest. An early comprehensive surgical evaluation may avoid these adverse effects which can be a direct result of epileptic syndrome.
The preoperative workup begins with a thorough history and physical examination. Almost all patients have significant preoperative hemiparesis related to the abnormal hemisphere. Preoperative hemianopsia is assessed. Neuropsychological evaluation of older pediatric patients provides a baseline for postoperative comparison and can provide insight regarding the functional impairment that may result from surgical intervention.
Evaluation of speech dominance is another important consideration. Language lateralization most likely occurs by age 6. Careful determination of language dominance is especially important after this age because older children are less likely to achieve contralateral function transfer after surgery, resulting in higher risk of a permanent language deficit.
Magnetic resonance (MR) imaging localizes the epileptogenic hemisphere and excludes structural abnormalities in the intact hemisphere. All patients should undergo a video electroencephalography (EEG) recording in an attempt to confirm the epileptogenic hemisphere and exclude any potential seizure activity originating from the intact hemisphere. Any evidence of contralateral abnormality on imaging or electrical monitoring is associated with significant functional decline after surgery.
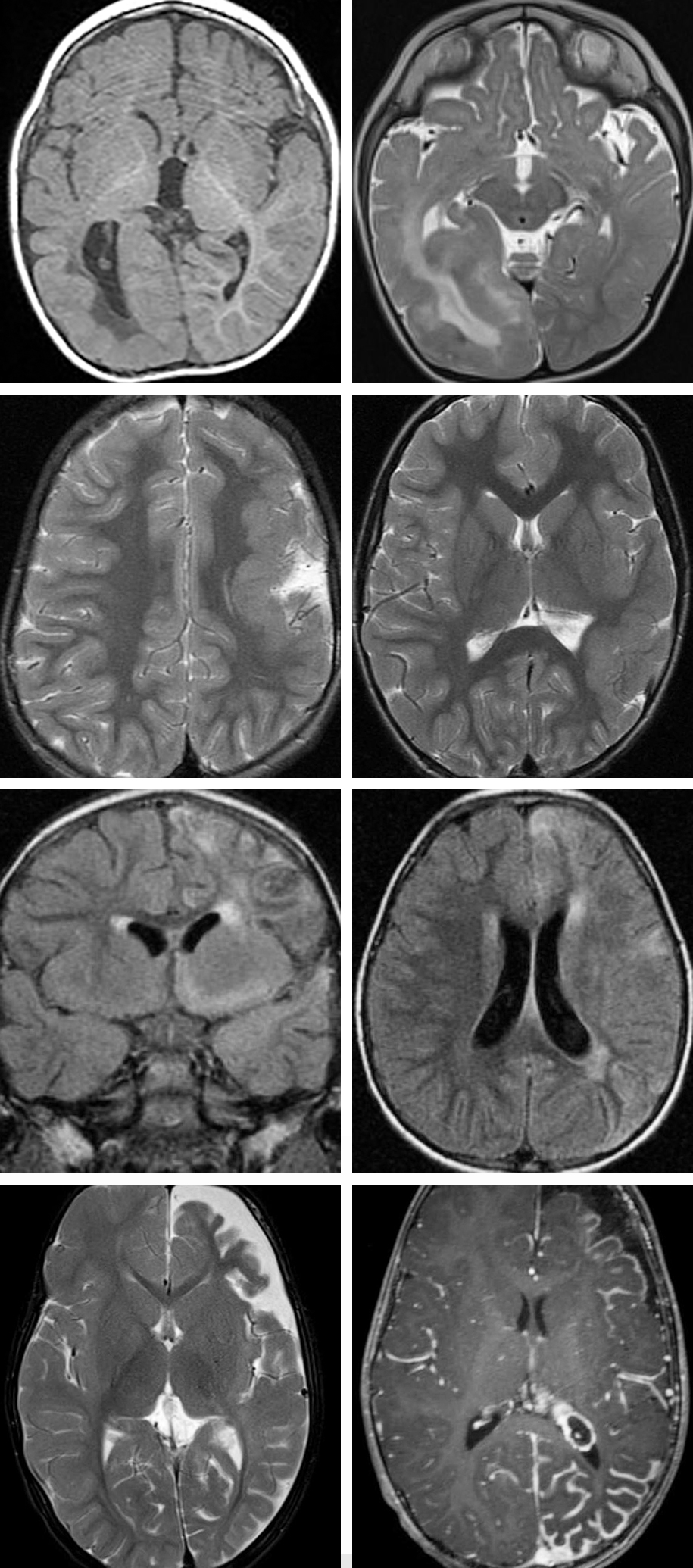
Figure 1: Right-sided diffuse cortical malformation of development with mild hemimegalencephaly is apparent (top row). Note the periventricular changes; ectopic gray matter may also be found in the periventricular area. These abnormalities are commonly most apparent in the posterior hemisphere. The second row demonstrates left-sided perisylvian pachygyria. The third row shows images consistent with left-sided Rasmussen’s encephalitis proven on biopsy; T2 signal abnormalities associated with hemispheric atrophy are present. The bottom row represents imaging stigmatas of Sturge-Weber syndrome, including left-sided frontoparietal and temporal hemiatrophy. Occipitotemporal leptomeningeal thickening and enhancement is consistent with pial angiomatosis.
Preoperative Considerations
The length of surgery and blood loss should be minimized because pediatric patients have a limited reserve of blood volume. A central venous catheter is strongly recommended as it provides rapid repletion of intravascular volume. The length of surgery should also be minimized to decrease the surgical stress on the patient; the technical challenges of this procedure can lead to lengthy operative sessions. Intraoperative image guidance based on MR imaging is beneficial.
Surgical Technique for Hemispheric Deafferentation
Multiple variations of hemispherotomy have been described, and the most common variations are peri-insular hemispherotomy, modified peri-insular hemispherotomy, and vertical parasagittal hemispherotomy. All hemispherotomy techniques have four commonalities: resection of medial temporal structures, interruption of the fibers forming the internal capsule and corona radiata, transventricular corpus callosotomy, and disruption of the frontal horizontal fibers.
The surgical technique described below is a modification of the procedure first developed by Schramm and colleagues in an attempt to decrease operative time and blood loss, remove less brain tissue, perform a smaller craniotomy, and achieve similar seizure relief when compared with functional hemispherectomies. I advocate this technique over other hemispherotomy techniques because access to the lateral ventricle is gained through the temporal horn, a technique that is common and familiar to epilepsy surgeons.
Operative Anatomy
Hemispherotomy is a technically challenging operation that requires a thorough understanding of three-dimensional cerebral anatomy to ensure a safe and thorough hemispheric disconnection.
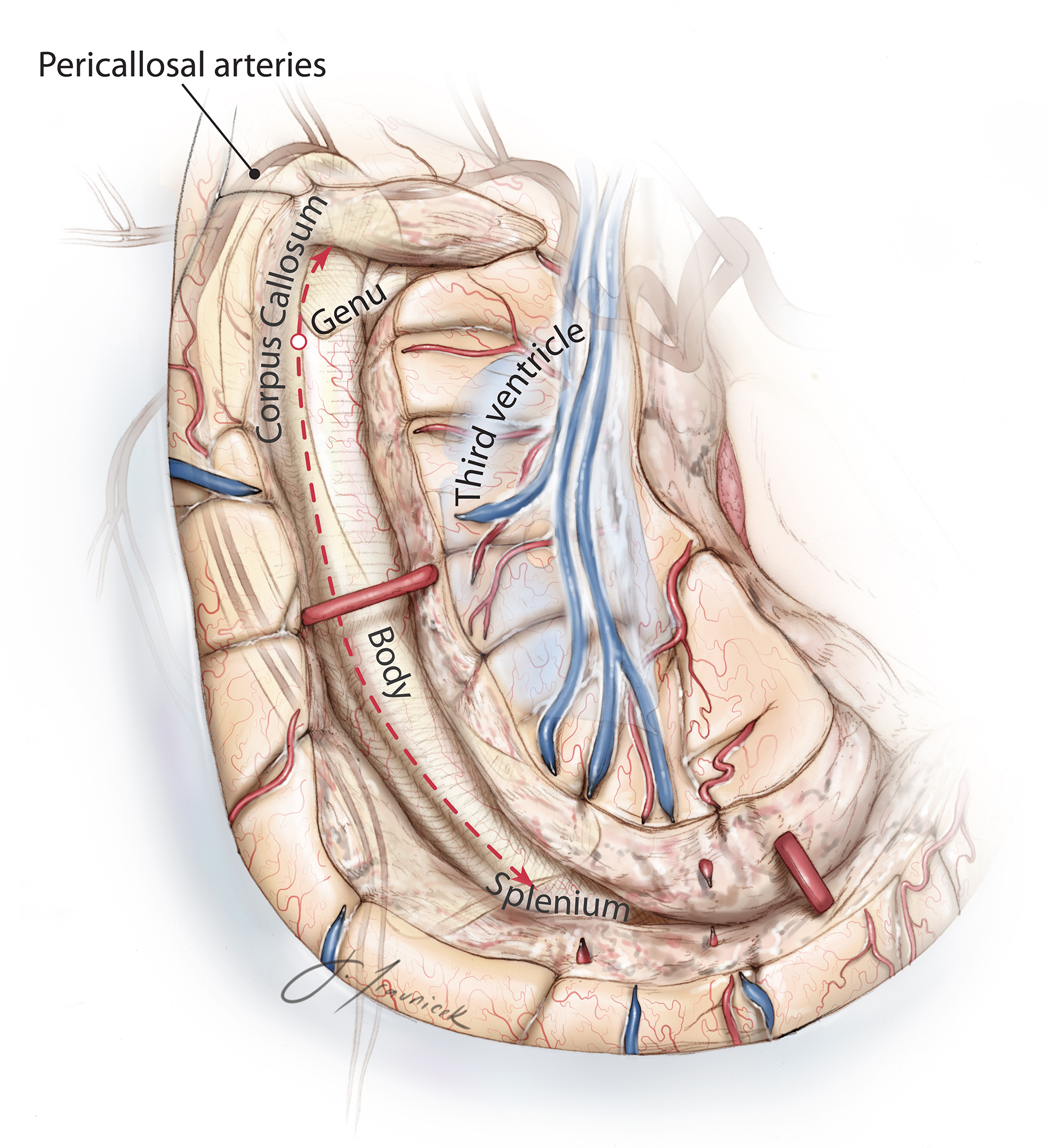
Figure 2: A lateral overview of a C-shaped peri-insular hemispherotomy in shown (top image). The entire lateral ventricle is unroofed. The yellow arrow points at the resection of the medial temporal structures; the blue arrow indicates the transventricular corpus callosotomy; and the red arrow marks the disruption of the horizontal frontal fibers. A coronal view of a hemispherotomy is also included (bottom photo). The white arrow shows the area corresponding to the interruption of the internal capsule from the operculum, whereas the black arrow indicates the transventricular corpus callosotomy. The red line indicates the insulectomy, and the blue arrow indicates the interruption of the temporal stem and resection of the medial temporal structures. c = claustrum; cc = corpus callosum; cg = cingulate gyrus; cn = caudate nucleus; ec = external capsule; fg = fusiform gyrus; phg = parahippocampal gyrus; T1 = superior temporal gyrus; T2 = middle temporal gyrus; T3 = inferior temporal gyrus (images used with permission from Morino et al. Anatomical analysis of different hemispherotomy procedures based on dissection of cadaveric brains. J Neurosurg. 2002;97:423-431).
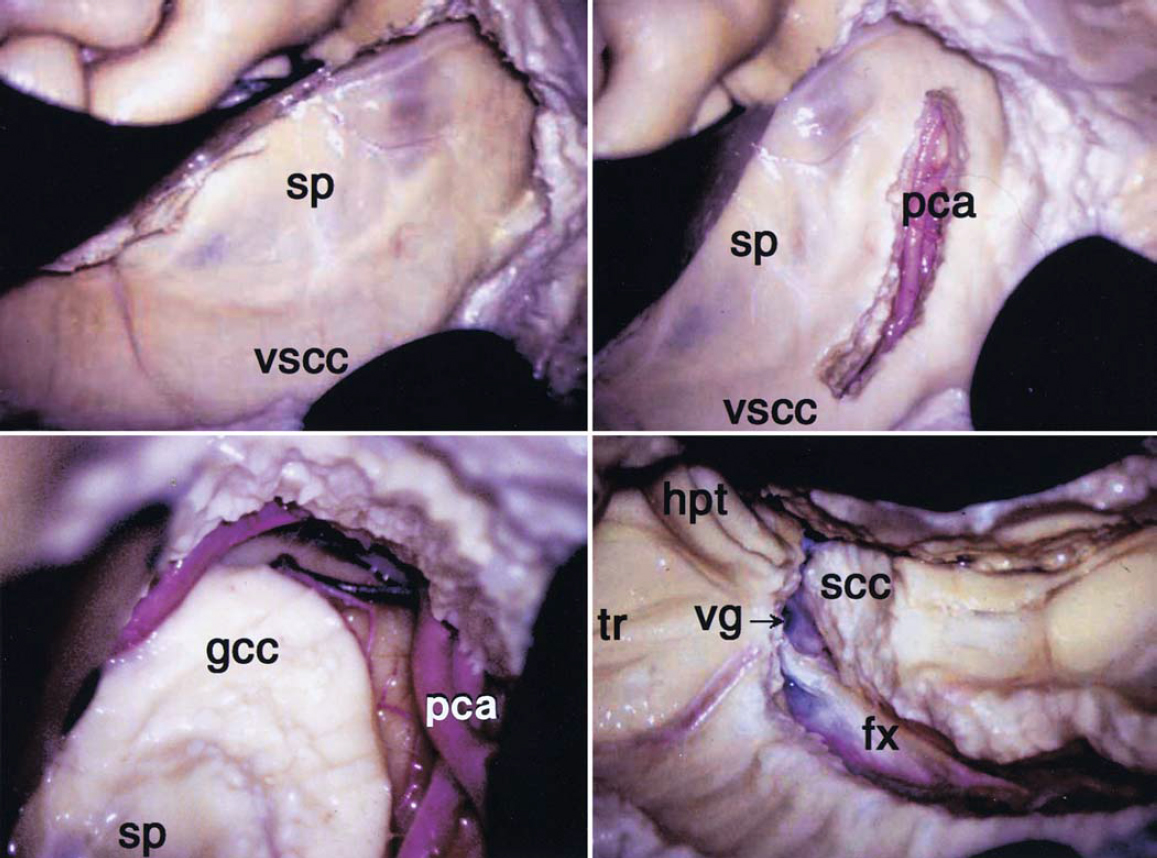
Figure 3: After a temporal lobectomy and removal of the medial structures, a C-shaped corticotomy is performed over the lateral hemisphere, starting from the roof of the temporal horn. The corticotomy outlines the lateral ventricular system, including the atrium; all components of the lateral ventricle are unroofed. This maneuver interrupts the internal capsule. In these images, the left side of the septum pellucidum (sp) and the ventral surface of the corpus callosum (vscc) may be recognized after entering the lateral ventricle (left upper image). A callosotomy is conducted along the course of the pericallosal artery (pca)(right upper image). Following the pericallosal artery toward the base of the frontal lobe, I complete transection of the genu of the corpus callosum (gcc), but stay a few millimeters away from the foramen of Monro (left lower image). The pericallosal artery is also pursued and skeletonized posteriorly so that the splenium of the corpus callosum (scc) is transected at the transition point between the hippocampus and fornix (right lower image). The posterior aspect of the falx can also be a reliable landmark to finish the callosotomy. After the splenium is transected, the vein of Galen (vg) can be found at the medial side of the splenium. fx = falx; hpt = hippocampal tail; tr = trigone (images used with permission from Morino et al. Anatomical analysis of different hemispherotomy procedures based on dissection of cadaveric brains. J Neurosurg. 2002; 97: 423-431.)
Figure 4: The horizontal frontal fibers (hff) are transected via continuation of the subcortical dissection starting from the point where the genu callosotomy left off and following the outline of the sphenoid wing (top photo). The fiber dissection method demonstrates that the horizontal frontal fibers include the occipitofrontal fasciculus (off) and uncinate fasciculus (uf)(bottom photo). ac = anterior commissure; cr = corona radiata; gp = globus pallidus; p = putamen; sw = sphenoid wing (images used with permission from Morino et al. Anatomical analysis of different hemispherotomy procedures based on dissection of cadaveric brains. J Neurosurg. 2002;97: 423-431.)
MODIFIED PERI-INSULAR HEMISPHEROTOMY
The patient is positioned supine on the operating table. The single pediatric pin of the skull clamp is placed behind the ipsilateral ear above the superior nuchal line, while the double pin is placed on the contralateral superior temporal line.
Figure 5: These skull clamp configurations allow generous exposure of the ipsilateral hemicranium. Skull clamps are generally used in children who are 2 years of age or older; the clamp allows the use of neuronavigational systems. I find neuronavigation helpful especially during intraventricular dissection to guide the extent of genu callosotomy and the medial extent of frontal fibers tractotomy. A large gel pad is used under the ipsilateral shoulder to avoid significant torsion of the neck while allowing the head to turn about 70 degrees away from the midline. The skin incision is a standard question mark incision centered on the peri-insular region.
INTRADURAL PROCEDURE
The dissection begins with a standard temporal lobectomy with removal of the medial structures. The details of this procedure are included in the Anteromedial Temporal Lobectomy chapter. The temporalis muscle is reflected forward in a single layer with the skin flap, and a craniotomy is performed to expose the peri-insular region and temporal lobe. Following the craniotomy, the dura is opened and reflected in the direction of the skin flap.
Figure 6: The temporal lobectomy is generous and includes an extended neocortical resection (6 cm from the temporal tip) and removal of the medial temporal structures (amygdala and hippocampus). This resection provides the surgeon with a conduit to access the temporal horn. Although I have employed the temporal lobectomy to expand the operative corridor into the temporal horn, especially among patients with hemimegalencephaly and associated small ventricles, this lobectomy is not routinely necessary. The operator can create an infrasylvian window through a middle temporal corticectomy to reach the temporal horn.
Figure 7: Identification of the choroidal fissure (an especially important landmark) is critical because all structures located medial to the fissure belong to the diencephalon and should be protected throughout the procedure. The opercular portion of the supramarginal gyrus leading to the already resected middle temporal gyrus is then removed, therefore unroofing the atrium of the lateral ventricle. The location of the ventricles is outlined.
Care is taken to preserve large cortical branches of the MCA crossing the areas of cortical resection during the entire procedure to preserve distal cortices and minimize their resultant ischemia.
Figure 8: Next, the corticotomy is carried anteriorly with removal of portions of the postcentral, precentral, and inferior frontal gyri, thus exposing the body and frontal horn of the lateral ventricle. Note the viewing angle toward the junction of the septum pellucidum and corpus callosum (inset image). This angle is oblique and may be disorienting to the operator. Image guidance can assist with identification of the appropriate trajectory. Any injury to the contralateral hemisphere should obviously be avoided.
Figure 9: The corticotomy is continued anteriorly along the lateral wall of the frontal horn. Note the use of cottonoid patties to protect the center of the hemisphere bounded by the choroid plexus. Again, the larger bridging branches of the middle cerebral artery are preserved. This maneuver is imperative because sacrifice of these branches will lead to infarction of large territories and compromises the advantages of hemispherotomy in preserving disconnected blocks of brain.
Figure 10: The corpus callosum is now in view and the callosotomy may be performed. In this illustration, the location of the corpus callosum, anterior cerebral artery complex, and third ventricle have been overlaid at the depth of the surgical field to provide an appreciation of the complex operative anatomy and the working angles necessary for callosotomy.
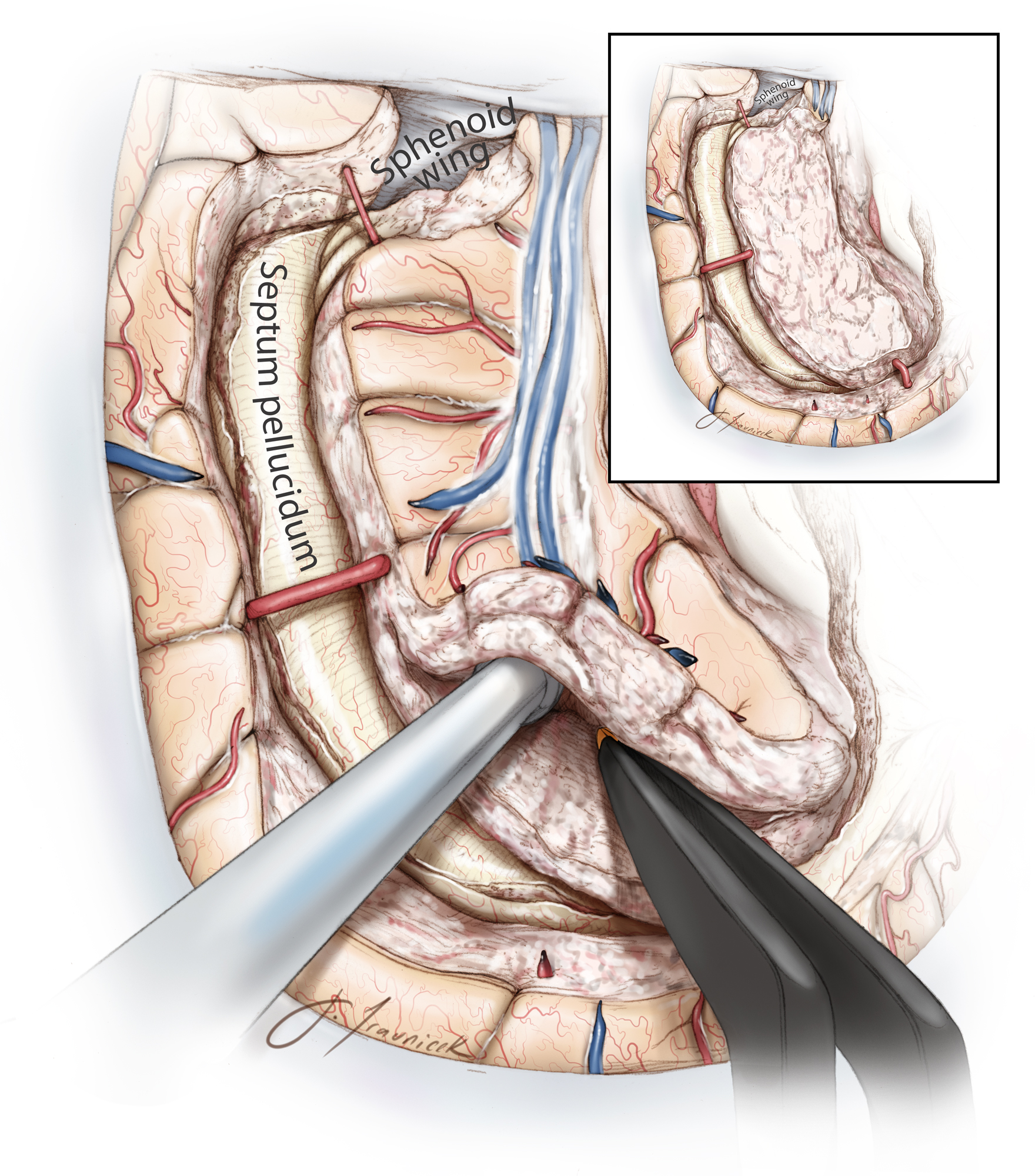
Figure 11: The junction of the septum pellucidum and the genu of corpus callosum serves as a landmark for starting the callosotomy, which is performed 4 to 5 mm from the midline due to the working angle provided by the transcortical transventricular access. During this step, neuronavigation can help confirm the correct plane of disconnection. I have not used ultrasound guidance because of the narrow transventricular operative corridor. The dissection is carried through the corpus callosum until the pericallosal arteries are identified within the callosal sulcus, just inferior to the cingulate gyrus. I find it helpful to place a small incision in the coronal plane to identify the artery, and then I continue with an incision in the parasagittal plane to start the callosotomy.
These illustrations idealize the operative view, but in reality, the operator’s working depth is long.
Identification of the pericallosal segment ensures that the dissection has not crossed the midline, placing the contents of the contralateral intact hemisphere at risk. Following identification of the proximal A2 branches, I extend the genu callosotomy anteriorly while pursuing the A2 branches through their encasing arachnoid membranes. Due to significant thickness of the genu, this arterial landmark and neuronavigation guidance are important to facilitate surgical orientation. Genu callosotomy is stopped at a coronal plane about 5 mm short of the foramen of Monro to avoid injury to the diencephalic structures.
Figure 12: The callosotomy is then carried posteriorly through the body and splenium of the corpus callosum. Aside from the pericallosal arteries, I also use the falx as an important landmark that may be pursued while carrying out the body and splenium callosotomy. This landmark is especially effective during transection of the splenium because small distal A3 and A4 branches are not easily identifiable within the interhemispheric fissure.
Figure 13: Following completion of the callosotomy, I turn my attention to the posterior extent of the hippocampectomy where the horizontal edge of the tentorium is exposed and the tail of the hippocampus transected subpially. White mater dissection through the Calcar avis and the medial wall of the atrium allows me to pursue the horizontal edge of the tentorium subpially, leading to its ascending segment.
Figure 14: The disconnection proceeds superiorly following the ascending aspect of the tentorium until the last sections of the splenium, calcar avis, tail of the hippocampus, crus fornices, cuneus, and precuneus are disconnected during this step. Pursuing the junction of the tentorium joining the falx cerebri further helps the surgeon confirm complete white matter disconnection along the splenium. The horizontal and ascending segments of the tentorium as well as the falx cerebri were imported on the latter sketches to aid the operator’s orientation.
Figure 15: The posterior edge of the falx continues to guide the surgeon to reach the tentorium. Care should be taken to continue subpial dissection during this transection and to preserve branches of the posterior cerebral artery crossing through the medial and inferior arachnoid membranes. The dissection within the atrium of the lateral ventricle must be performed posterior to the choroid plexus to ensure preservation of the thalamus.
Figure 16: Once the posterior deafferentation is complete, the basal surface of the frontal lobe is disconnected. For this portion of the dissection, the lesser wing of the sphenoid bone is used as a landmark to guide the tractotomy of the basal frontal fibers. I limit my dissection to the lateral part of the clinoid to avoid injuring the hypothalamus. Anterior cerebral arteries are skeletonized to the level of the skull base to ensure adequate transection. At the completion of this step, the operator exposes the frontal horn of the lateral ventricle and the area of previous genu callosotomy through the frontal lobe. The bony clinoid has been illustrated here at the depth of the field and the green arrow outlines the route of disconnection.
Figure 17: Finally, the insular cortex is removed while the middle cerebral artery branches are protected.
It is also important to confirm visualization of the pia on the medial side of all of the disconnection routes to ensure a complete hemispheric deafferentation before closure.
I do not routinely place a catheter within the ventricle for temporary postoperative drainage unless immaculate hemostasis is impossible. The ventricles are copiously irrigated to remove debris.
Case Example
Figure 18: The steps involved with a right-sided hemispherotomy procedure are shown. The images of the upper row show completion of the temporal lobectomy, exposure of the lateral ventricle via a C-shaped corticotomy over the roof of the ventricle, and identification of the pericallosal arteries during the initial stages of the genu callosotomy. The images in the middle row demonstrate the steps in using the falx (left) and tentorium (right) as road maps to complete the splenium callosotomy. The images in the last row show the use of the sphenoid wing and clinoid process as a map to perform the subcortical frontal tractotomy (lower left image). The resection of the insula is complete (lower right image).
Techniques of modified peri-insular hemispherotomy for multilobar cortical dysplasia.
Complications
Residual Seizures and Potential Reasons for Suboptimal Disconnection
Residual seizures most often occur when deafferentation is incomplete. Most commonly, this is the result of an incomplete disconnection of the corpus callosum and, more specifically, the genu and splenium. The possibility of this complication may be minimized by exposure of the pericallosal arteries and falx cerebri as described above. The use of an intraoperative EEG on the ipsilateral occipital lobe and the contralateral hemisphere may help with confirmation of the callosotomy.
Inadequate basal frontal lobe disconnection and insular resection have also been implicated in seizure recurrence. I define the extent of the basal subcortical dissection along the clinoid to ensure complete disconnection. I have also modified the previous techniques to pursue the horizontal and ascending edges of the tentorium to ensure complete disconnection in the region. Partial genu callosotomy is another potential reason for inadequate hemispheric disconnection; neuronavigation along with the route of A2 branches provides an adequate surgical roadmap.
Patients suffering from recurrent seizures should undergo high-resolution MR imaging and diffusion tensor imaging to assess the completeness of hemispheric deafferentation. I do not routinely obtain a postoperative MRI. Due to the working space limitations within the ventricles, patients with hemimegalencephaly are likely to have a higher risk of inadequate disconnection.
Hydrocephalus
Although hydrocephalus occurs less commonly after hemispherotomy than hemispherectomy, it still complicates approximately 2% to 15% of operations. Hydrocephalus necessitating permanent shunt placement is more likely to occur when the underlying pathology is hemimegalencephaly or another multilobar cortical dysplasia.
Outcomes
A number of studies have confirmed the effectiveness of hemispherotomy in appropriately selected patient populations. Functional hemispherotomy or peri-insular hemispherotomy is associated with an 80% chance of the cessation of disabling seizures. The remainder of the patients who do not attain complete cessation of their seizures benefit at least from a worthwhile improvement compared with their preoperative status.
Pearls and Pitfalls
- Hemispherotomy is an effective treatment for pediatric epilepsy resulting from marked dysfunction of a single cerebral hemisphere. Although technically more challenging than similar operations, the procedure has the potential to provide adequate relief from seizures while limiting postoperative complications.
DOI: https://doi.org/10.18791/nsatlas.v7.ch04
This chapter was previously presented in a similar format as part of the following publication:
Kovanda TJ, Rey-Dios R, Travnicek J, Cohen-Gadol AA. Modified peri-insular hemispherotomy: operative anatomy and technical nuances. J Neurosurg Pediatr. 2014;13:332-338. PMID: 24410122. Permission for the use of the text was granted.
References
Battaglia D, Chieffo D, Lettori D, Perrino F, Di Rocco C, Guzzetta F. Cognitive assessment in epilepsy surgery of children. Childs Nerv Syst. 2006;22:744-759.
Centeno RS, Yacubian EM, Sakamoto AC, Ferraz AF, Junior HC, Cavalheiro S. Pre-surgical evaluation and surgical treatment in children with extratemporal epilepsy. Childs Nerv Syst. 2006;22:945-959.
Cook SW, Nguyen ST, Hu B, Yudovin S, Shields WD, Vinters HV, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg. 2004;100:125-141.
Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:952-959.
Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA. 1928;90:823-825.
De Ribaupierre S, Delalande O. Hemispherotomy and other disconnective techniques. Neurosurg Focus. 2008;25:E14.
Delalande O, Bulteau C, Dellatolas G, Fohlen M, Jalin C, Buret V, et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60:ONS19-32; discussion ONS32.
Heinz ER, Heinz TR, Radtke R, Darwin R, Drayer BP, Fram E, et al. Efficacy of MR vs CT in epilepsy. Am J Roentgenol. 1989;152:347-352.
Kim DL, Osburn LL, Cohen-Gadol AA. A novel method for confirmation of hemispheric disconnection during hemispherotomy surgery. Pediatr Neurosurg. 2010;46:71-75.
Krynauw RA. Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13:243-267.
Limbrick DD, Narayan P, Powers AK, Ojemann JG, Park TS, Bertrand M, et al. Hemispherotomy: efficacy and analysis of seizure recurrence. J Neurosurg Peds. 2009;4:323-332,
Marras CE, Granata T, Franzini A, Freri E, Villani F, Casazza M, et al. Hemispherotomy and functional hemispherectomy: indications and outcome. Epilepsy Res. 2010;89:104-112.
McKenzie K: The present status of a patient who had the right cerebral hemisphere removed. JAMA. 1938;111:168.
Morino M, Shimizu H, Ohata K, Tanaka K, Hara M. Anatomical analysis of different hemispherotomy procedures based on dissection of cadaveric brains. J Neurosurg. 2002;97:423-431.
Oppenheimer DR, Griffith HB. Persistent intracranial bleeding as a complication of hemispherectomy. J Neurol Neurosurg Psychiatry. 1966;29:229-240.
Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983; 10:71-78.
Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509-515; discussion 515-516,
Shimizu H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia. 2005;46 (Suppl 1):30-31.
Taugher B, Richards M. Functional hemispherectomy. Axone (Dartmouth, N.S.) 1992;14:29-32.
Vadera S. Moosa AN. Jehi L. Gupta A. Kotagal P. Lachhwani D. Wyllie E. Bingaman W. Neurosurgery. 2012;71:388-392; discussion 392-393.
Van Schooneveld MM, Braun KP: Cognitive outcome after epilepsy surgery in children. Brain Dev. 2013;35:721-729.
Villemure JG, Daniel RT. Peri-insular hemispherotomy in paediatric epilepsy. Childs Nerv Syst. 2006;22:967-981.
Wen HT, Rhoton AL Jr., de Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M, et al. Microsurgical anatomy of the temporal lobe: part 1: mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999;45:549-591; discussion 591-542.
Wen HT, Rhoton AL, Jr., Marino R Jr. Anatomical landmarks for hemispherotomy and their clinical application. J Neurosurg. 2004;101:747-755.
Wyllie E. Surgical treatment of epilepsy in pediatric patients. Can J Neurol Sci. 2000;27:106-110.
Please login to post a comment.