Surgery for Acoustic Neuroma: Translabyrinthine Approach
This is a preview. Check to see if you have access to the full video. Check access
Resection of an Acoustic Neuroma through the Translabyrinthine Approach
Please note the relevant information for patients with acoustic neuroma is presented in another chapter. Please click here for patient-related content.
For general considerations, clinical presentation, and evaluation of vestibular schwannomas, please refer to the Retrosigmoid Approach for Acoustic Neuroma chapter.
Indications for the Translabyrinthine Approach
Vestibular schwannomas (VSs) have been resected via the translabyrinthine (TL), middle cranial fossa (MCF), or retrosigmoid (RS) approaches, based on their size/location of the bulk of the tumor, status of the patient’s hearing, and the preference of the operating surgeon. Other factors that play a role in selection of the approach include the patient’s age and overall health status, anatomy of the vestibule and cerebellopontine (CP) angle, and the involvement of the brainstem and the internal auditory canal (IAC). The RS approach is favored by neurosurgeons and offers an opportunity for hearing preservation while tackling different size tumors via the panoramic view provided by the CP angle.
The TL osteotomy precludes the possibility of hearing preservation, but allows removal of almost any size tumor with early identification of the facial nerve; some colleagues argue that the TL route also allows good preservation of all cranial nerves and reduces retraction on the cerebellum. Indications for the TL route include larger tumors and a preoperative lack of serviceable hearing; our neuro-otology colleagues traditionally have favored the TL option.
VSs have been stratified into these tumor size/location categories: intracanalicular, <1.5 cm (small), 1.5 to 3.0 cm (medium), and >3.0 cm (large). Some surgeons claim that the RS corridor may result in less neurologic dysfunction during resection of intracanalicular tumors than the MCF route, but the MCF option may be superior to the RS approach for hearing preservation in patients with tumors smaller than 1.5 cm. The RS pathway is potentially associated with less dysfunction than the MCF or TL approaches for tumors 1.5 to 3.0 cm.
Postoperative headache tends to be more prevalent after the RS than the TL route. The risk of cerebrospinal fluid (CSF) leak is greater following an RS craniotomy than both the MCF and TL approaches. Some studies report that the incidences of mortality, major noncranial nerve neurologic complications, residual tumor, tumor recurrence, and dysfunction of other cranial nerves are not significantly different across the various approaches. There is no class I data to strongly support one approach over another.
In summary, the MCF corridor seems to be safest for hearing preservation in patients with smaller tumors. The RS craniotomy is likely the most versatile corridor for most tumor sizes, but it is associated with a higher risk of postoperative pain and CSF fistula. Finally, the TL osteotomy is associated with hearing loss, but may be beneficial for patients with large tumors and poor preoperative hearing.
The TL approach can be expanded via the use of the transtentorial routes to reach a wider breadth of the posterior fossa, tentorial incisura, and supratentorial space for handling other related tumors. The addition of the temporal craniotomy with preservation of the labyrinth creates the extended posterior petrosal approach.
Preoperative Considerations
The TL corridor has been used for CP angle meningiomas, facial and trigeminal schwannomas, cholesteatomas, chordomas, and even occasionally for intraaxial lesions such as pontine cavernous malformations.
Preoperative computed tomography (CT) can definitively evaluate the affected bone; the addition of a CT angiogram can elucidate the relevant anatomy of the dural venous sinuses to the temporal bone. Anteriorly located sigmoid sinuses can limit the area of the Trautmann triangle or the presigmoid dura, restricting the operative corridor. Furthermore, a high-riding jugular bulb can restrict access to the inferior extent of the tumor.
Active otitis media is a contraindication for the use of the TL route because an infected operative field could be crossed. Facial nerve electromyography (EMG) for mapping the nerve is considered the standard of care. Electrodes are placed in the frontal and orbicular muscles. Brainstem auditory evoked responses (BAERs) are not used due to the expected hearing loss while somatosensory evoked potentials (SSEPs) are monitored. My otolaryngology colleagues play an important role during performance of the osteotomy.
I place a lumbar drain unless the patient is suffering from obstructive hydrocephalus that requires an external ventricular drain. The gradual drainage of CSF during the extradural osteotomy and labyrinthectomy protects the dura and the sigmoid sinus and prevents cerebellar herniation during the dural opening.
Operative Anatomy
The anatomy of the temporal bone is an important prerequisite for performance of the TL osteotomy. It is mandatory that surgeons practice temporal bone drilling in the microsurgical laboratory.
Click here to view the interactive module and related content for this image.
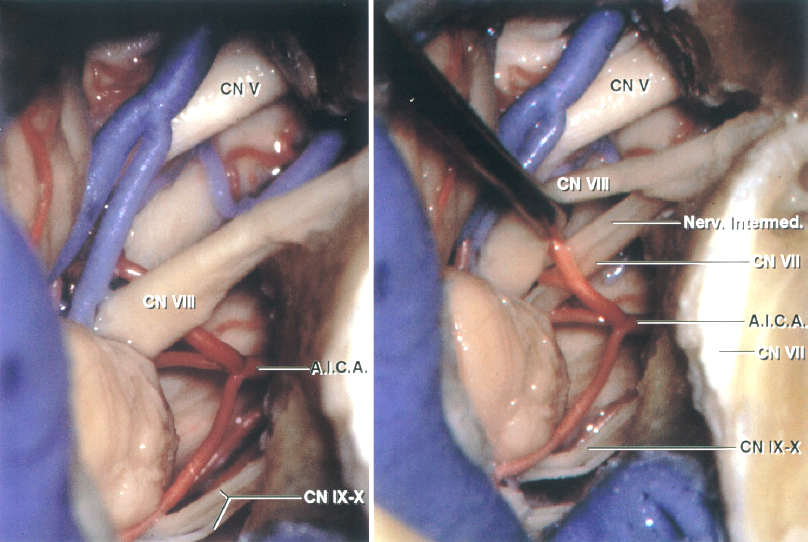
Figure 1: The TL approach is summarized. The spine of Henle at the posterosuperior margin of the external meatus (inset image) is a superficial landmark that estimates the deep location of the lateral semicircular canal and the tympanic segment of the facial nerve. The mastoidectomy has been completed and the dural venous sinuses and the facial nerve are skeletonized while a thin layer of bone is left over them. The semicircular canals are apparent. The dura, bounded by the sigmoid and superior petrosal sinuses, the jugular bulb, and the labyrinth, is the Trautmann triangle (left upper image). The tympanic segment of the facial nerve courses between the lateral canal and the stapes in the oval window, and then turns inferiorly as the mastoid segment (right upper image). The semicircular canals and vestibule have been resected and the dura covering the IAC has been incised to expose the cranial nerve (CN) VII/VIII complex (left lower image). The dura has been further resected to reveal the CP angle. The limits of the exposure include an anteriorly positioned sigmoid sinus, a high-riding jugular bulb, or a low middle fossa plate. The jugular bulb may be situated as high as the posterior wall of the IAC and be found at the posterior meatal wall during the TL and RS approaches. (Images courtesy of AL Rhoton, Jr.)
Click here to view the interactive module and related content for this image.
Figure 2: The intradural anatomy of the CP angle via the TL approach is shown. The jugular bulb may obstruct the view of the lower cranial nerves entering the jugular foramen (left image). CN VIII has been mobilized to expose the facial nerve. (Images courtesy of AL Rhoton, Jr.)
RESECTION OF VESTIBULAR SCHWANNOMA VIA THE TRANSLABYRINTHINE APPROACH
The patient is positioned supine on the operating room table. The thorax is slightly elevated to promote venous drainage.
Figure 3: The head is rotated toward the opposite side. If the patient has a supple neck, up to 70 degrees of rotation may be applied, whereas if the patient has limited neck mobility, a shoulder roll can compensate for the lack of rotation. The steps for scalp dissection and anterior reflection of the suboccipital muscles are shown in the inset images.
The head clamp is positioned so that the pins stay away from the surgical field, although neuro-otologists commonly perform the operation with the patient in the supine position and a gel ring to stabilize the head. The abdomen is prepared for harvesting fat graft to facilitate closure of the dural defect at the end of the operation.
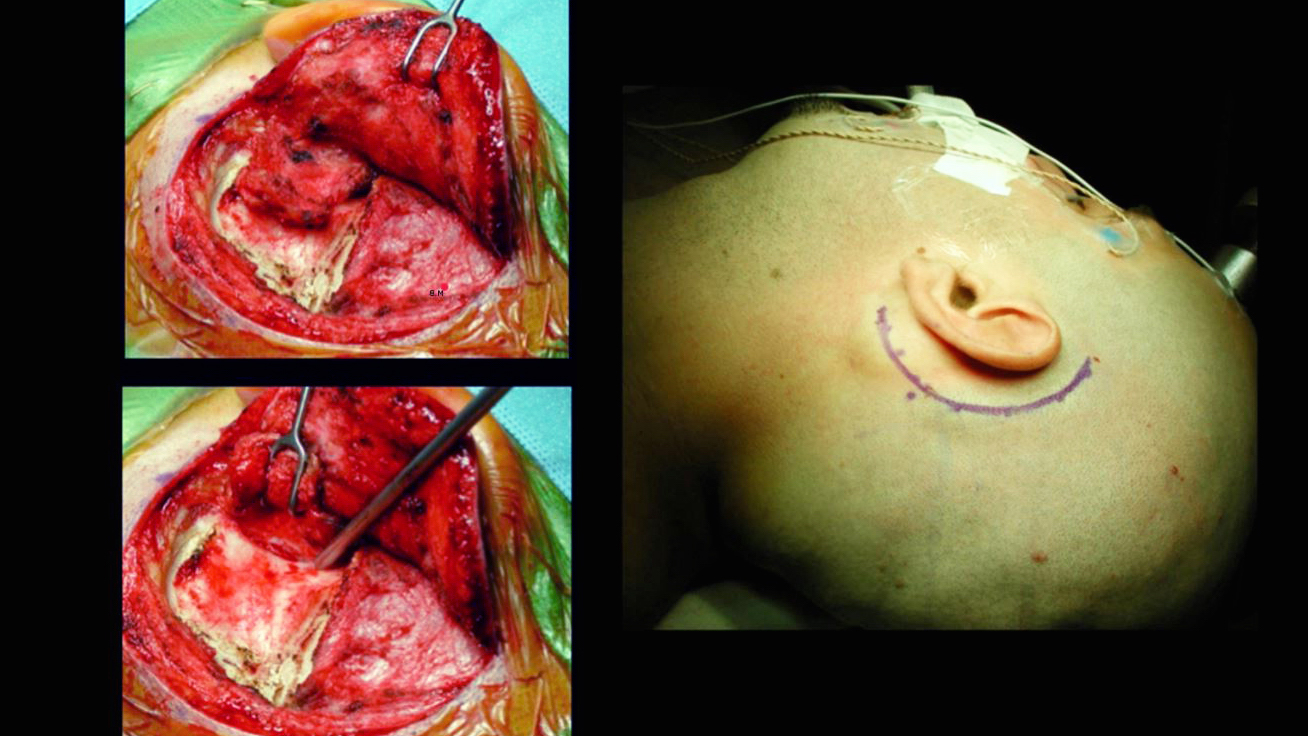
Figure 4: The traditional incision for the TL approach is a curvilinear one beginning approximately 1 cm superior to the pinna, proceeding posterior to the sigmoid sinus and ending at the mastoid tip. The incision on the mastoid tip may be extended more inferiorly if the scalp layers are thick and more aggressive reflection of the myocutaneous flap is required.
Figure 5: I prefer to reflect a single myocutaneous flap anteriorly. Alternatively, the temporal fascia is incised along the edges of the incision and separated from the muscle. This fascial flap is further developed beyond the superior nuchal line in continuity with the sternocleidomastoid muscle, which is released from its insertion on the mastoid and occipital bones. This technique provides a large vascularized fascial flap that may be used for reconstruction of the dural defect at the time of closure. The soft tissue flap is mobilized until the spine of Henle can be seen. The external auditory canal should not be entered.
Mastoidectomy
The translabyrinthine approach begins with a complete mastoidectomy. The first step is decortication of the mastoid surface using a high-speed cutting burr.
Figure 6: Troughs are created to outline the superior and anterior limits of the mastoidectomy. The superior trough extends from the root of the zygoma to the asterion, just inferior to the infratemporal line. The anterior trough extends from the posterior rim of the external auditory meatus down to the level of the mastoid tip. The posterior trough overlaps with the edges of the sigmoid sinus.
The mastoid air cells are removed within these troughs. Drilling should take place at an even depth throughout. The cortical bone covering the sigmoid sinus is identified as the air cells are removed. My neuro-otology colleagues usually perform this part of the procedure.
Figure 7: Once the sigmoid sinus is skeletonized, additional air cells anterior to the sigmoid sinus are removed to expose the presigmoid dura of the middle and posterior fossae. Approximately 1 to 2 cm of dura over the posterior fossa, posterior to the sigmoid sinus, is also exposed. Small islands of thin cortical bone are left on the venous sinus to minimize any injury to this structure. This bone removal is continued until the middle fossa dura is completely exposed. Successively smaller cutting and diamond burrs are used as the mastoidectomy proceeds.
The next step is to identify the antrum by following the middle fossa plate and the posterior external auditory canal wall through the remaining superior mastoid air cells. The antrum is located about 15 mm deep to the spine of Henle.
The lateral semicircular canal is located deep to the antrum. This canal is the basic landmark in temporal bone surgery. Once its position is known, the depth and three-dimensional relationship of the facial nerve and posterior/superior semicircular canals can be identified.
The sigmoid sinus is followed inferiorly and the air cells of the mastoid tip are drilled away, exposing the inferior segment of the sigmoid sinus and the digastric ridge. The facial nerve courses from the inferior edge of the lateral semicircular canal to the stylomastoid foramen, located just anterior to the digastric ridge. The facial nerve is embedded in the cortical bone of the facial (or fallopian) canal.
Labyrinthectomy
CSF drainage via the lumbar drain assists with mobilization of the dura. I remove 10 cc aliquots with maximum volume of about 60 cc.
Figure 8: After the mastoidectomy is complete, the labyrinthectomy begins. First, bone removal is continued over the sinodural angle along the superior petrosal sinus, exposing the Trautmann triangle. This opening is gradually deepened and widened until the labyrinth is encountered. The lateral and posterior semicircular canals are removed and the common crus of the superior and posterior semicircular canals is identified.
The superior semicircular canal is followed to its ampulla. The vestibule is opened, and the facial nerve is skeletonized from its genu toward the stylomastoid foramen. The bone lateral to the facial nerve may be left in place while removal of the bone posterior to the facial nerve will improve the intradural working angles. These last steps of bone removal are performed using a diamond burr. After the facial nerve has been adequately skeletonized, the superior vestibular nerve entering the vestibule can be seen.
After removal of the labyrinth to the level of the vestibule, the IAC is identified and skeletonized. This maneuver begins along the superior petrosal sinus; bone removal is widened in all directions toward and around the IAC. The dura of the posterior fossa and IAC are exposed; care is taken to leave a thin eggshell of bone over the IAC.
The osteotomy is continued inferior to the labyrinth until the jugular bulb is found. This maneuver achieves the inferior extent of the exposure. Next, bone removal proceeds posterior to the IAC, where the vestibular aqueduct and the endolymphatic sac are encountered and removed. Final steps of the osteotomy involve bone removal around the inferior aspect of the IAC while ample irrigation fluid is used to avoid thermal injury to the facial nerve.
Once bone removal is complete over the inferior aspect of the IAC, I unroof the anterior/superior aspects of the canal. The goal is to expose the IAC 270 degrees around its circumference. At this final step, bone removal is slow and tedious because the facial nerve is situated just beneath the dura in this area. The superior lip of the porus acusticus is excised. The transverse crest and Bills’ bar are identified and allow distinction among the facial, superior, and inferior vestibular nerves.
Figure 9: The skeletonization of the IAC is shown (upper two photos). The dura is incised over the midportion of the IAC, and the incision extends around the porus acousticus superiorly and inferiorly (lower sketch). Great care is taken to avoid injury to the facial nerve and the petrosal vein. The dural flaps are retracted superiorly and inferiorly and cottonoids are placed between the tumor and the cerebellum posteriorly.
INTRADURAL PROCEDURE
Immaculate hemostasis over the epidural space facilitates efficient intradural microdissection and good visibility of the neurovascular structures.
Figure 10: After opening the dura, the dural flaps are mobilized using retention sutures. The posterior part of the tumor is uncovered. Opening of the arachnoid membranes releases additional CSF and relaxes the posterior fossa tension.
The facial nerve may occasionally lie on the posterior capsule of the tumor; this configuration should be noted via stimulation mapping. For details of facial nerve mapping and intradural tumor removal, please also refer to the Retrosigmoid Approach for Acoustic Neuroma chapter.
Figure 11: The techniques of resection for large tumors demand intracapsular decompression using an ultrasonic aspirator to develop safe extracapsular dissection planes without aggressive mobilization of the tumor. With small tumors, I can readily find the inferior and superior extracapsular dissection planes and proceed with tumor removal without significant tumor decompression.
Early identification of the facial nerve in the fundus of the IAC is the next important step and is confirmed via the stimulator probe. Further drilling of the fundus may be necessary to expose the nerve. The medial part of the fallopian canal is carefully exposed so that the labyrinthine segment of the facial nerve is reachable.
A fine right-angle hook can reach lateral to Bill’s bar and underneath the superior vestibular nerve. This maneuver allows gentle avulsion of the nerve out of its canal along with the attached tumor. Similarly, with large tumors, the two nerves inferior to the transverse crest (the inferior vestibular and the cochlear nerves) are pulled out from their canals along with a piece of the infiltrating tumor. Repeated stimulation confirms the health of the facial nerve. Early identification of the facial nerve at the lateral end of the IAC is one of the major advantages of the TL approach.
Figure 12: After transection or avulsion of the vestibular and/or cochlear nerves, extracapsular tumor mobilization reflects and peels off the tumor capsule from the facial nerve within the IAC in the medial direction. Stimulation mapping localizes the inferior and superior edges of the nerve within the IAC.
The most difficult part of dissection occurs at the porus where the tumor is most adherent to the nerve. Again, stimulation mapping identifies the course of the facial nerve at and medial to the porus. If the tumor is too adherent to the nerve, radical subtotal removal is advised. Next, I direct my attention to the medial tumor capsule near the brainstem. The nerve is then pursued from a medial-to-lateral direction and I meet the initial dissection plane of the tumor-facial nerve interface at the porus.
Aggressive handling and lateral reflection of the tumor is prohibited because the distal part of the facial nerve would be placed at risk since it is the only band holding the tumor to the porus. Intracapsular removal of the cisternal component of the tumor within the CP angle is necessary to reduce the bulk of the tumor and prepare for the next step.
The steps for dissection of the tumor within the CP angle are discussed in the Retrosigmoid Approach for Acoustic Neuroma chapter. As limits of identifiable dissection planes over the capsule are reached in one location at the brainstem, the dissection is diverted elsewhere.
Figure 13: The trigeminal nerve is identified and carefully released from the superior pole of the tumor capsule (top image). The cochlear nerve at the lower pole of the capsule is stimulated (bottom image) and transected in patients with larger tumors and nonserviceable hearing.
The anterior aspect of the tumor capsule contains small arteries and, more commonly, veins near the facial nerve. Bleeding from these vessels may be a significant source of annoyance. Gentle irrigation, application of tamponade using pieces of thrombin-soaked cotton, and operator’s patience are needed to avoid aggressive coagulation and suction that could easily injure the nerve.
Figure 14: The facial nerve is progressively dissected away from the anteroinferior surface of the tumor capsule.
Figure 15: After the tumor has been completely removed or subtotally resected (due to the potential risk to the facial nerve), the surgical field is inspected. Response of the facial nerve to stimulation of 0.07 mA at its exit zone near the brainstem is a good indicator of acceptable facial function after surgery.
Closure
The dura is approximated as much as possible. The middle ear is reached through the antrum, and the incus is removed. The eustachian tube can be found around the malleus and it is plugged with a piece of muscle.
The dural defect and middle ear are also packed with pieces of fat harvested from the abdomen. The mastoid air cells are thoroughly rewaxed. The bone defect is covered with a piece of titanium mesh and methyl methacrylate cranioplasty. The rest of the closure occurs in the anatomic layers.
Alternatively, a piece of fascia lata graft may be used to cover the dural defect. I believe that the globular feature of the fat is more effective in sealing CSF fistulas. The vascularized fascial flap prepared from the posterior aspect of the temporalis fascia may be rotated to fill the defect in the dura in the event of reoperations, infections, and previously radiated incisions.
The lumbar drain is used to drain 8cc/hour of CSF for 48 hours after surgery. Patients are mobilized as soon as possible.
Pearls and Pitfalls
- If the patient has nonserviceable hearing, the TL approach can be used to provide a direct route to the tumor.
- An extended mastoidectomy is followed by localization of the facial nerve near the lateral semicircular canal. Labyrinthectomy and skeletonization of the IAC lead to an early and adequate exposure of the distal facial nerve within the IAC.
Contributor: Andrew R. Conger, MD, MS
For additional illustrations of facial nerve repair, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of hypoglossal-facial anastomosis, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of microsurgical resection of an acoustic neuroma via a translabyrinthine approach, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of the retrolabyrinthine approach, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of the transcochlear approach, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of translabyrinthine approach, please refer to the Jackler Atlas by clicking on the image below:
For additional illustrations of tumor growth patterns, please refer to the Jackler Atlas by clicking on the image below:
DOI: https://doi.org/10.18791/nsatlas.v5.ch08.2
Some of the materials included in this chapter were previously described in the following articles:
Ansari SF, Terry C, Cohen-Gadol AA. Surgery for vestibular schwannomas: a systematic review of complications by approach. Neurosurg Focus. 2012;33:E14.
Kulwin CG, Cohen-Gadol AA. Technical nuances of resection of giant (>5 cm) vestibular schwannomas: pearls for success. Neurosurg Focus. 2012;33:E15.
References
Abdel Aziz KM, Sanan A, van Loveren HR, Tew JM, Keller JT, Pensak ML. Petroclival meningiomas: predictive parameters for transpetrosal approaches. Neurosurgery 2000;47:139-150; discussion 150-152.
Al-Mefty O. Operative Atlas of Meningiomas. Lippincott-Raven,, Philadelphia, PA; 1998.
Al-Mefty O, Fox JL, Smith RR. Petrosal approach for petroclival meningiomas. Neurosurgery 2002;22:510–517.
Brackmann D. Translabyrinthine/transcochlear approaches, in Sekhar LN, Janecka IP (eds): Surgery of Cranial Base Tumors. Raven Press, New York, NY; 1993:389-411.
Horgan MA, Anderson GJ, Kellogg JX, Schwartz MS, Spektor S, McMenomey SO, et al. Classification and quantification of the petrosal approach to the petroclival region. J Neurosurg 2000;1;93:108-112.
Horgan MA, Delashaw JB, Schwartz MS, Kellogg JX, Spektor S, McMenomey SO. Transcrusal approach to the petroclival region with hearing preservation. Technical note and illustrative cases. J Neurosurg 2001;94:660-666.
Miller CG, van Loveren HR, Keller JT, Pensak M, el-Kalliny M, Tew JM. Transpetrosal approach: surgical anatomy and technique. Neurosurgery 1993;33:461-469; discussion 469.
Sanna M. Temporal Bone--A Manual for Dissection and Surgical Approaches. Thieme, New York, NY; 2006:55-73.
Sincoff EH, McMenomey SO, Delashaw JB. Posterior transpetrosal approach: less is more. Neurosurgery 2007;60(2 Suppl 1):ONS 53–58; discussion ONS 58–59.
Tew JM, van Loveren HR, Keller JT. Atlas of Operative Microneurosurgery. Saunders, Philadelphia, PA; 1994-2001.
Please login to post a comment.