Anterior Petrosectomy Free
Click here to view related content for this model.
3D MODEL: Temporal Bone Anatomy: The instructions for use of this and other 3D models are as follows: Please use the full screen function for optimal visualization (by clicking on the arrows on the right lower corner of the model). To move the model in 3D space, use your mouse's left click and drag; to enlarge the object, use the mouse's wheel. The right click and drag function moves the model across the plane. Please click on “Select an annotation” link at the bottom of the window and “Show annotations” so that the anatomical labels become visible.
Technical Nuances for the Anterior Petrosal Approach
General Considerations
The middle fossa approach has been traditionally used for resection of small acoustic neuromas and meningiomas along the lip of the internal auditory canal (IAC). The natural extension of this approach has been the anterior petrosal approach (anterior petrosectomy) that involves expanded bony resection at the petrous apex to expose the upper petroclival region and ventrolateral brainstem.
Transpetrosal approaches have evolved to offer advantages over traditional operative corridors, such as retromastoid, middle fossa, and pterional trajectories for exposing lesions within the petroclival area. The terminology used to describe transpetrosal approaches is very nonspecific and sometimes confusing because different nomenclature has been used to report their modifications. However, if we systematically analyze all modified transpetrosal procedures, we find that they can be classified into two types:
- Anterior petrosectomy: removal of the petrous apex
- Posterior petrosectomy: removal of the petrous pyramid
More radical or extensive transpetrosal osteotomies that have been described in the literature are often combined approaches comprised of conventional craniotomies in conjunction with an anterior or posterior petrosectomy.
The petrous bone contains many critical structures including the cranial nerves VII/VIII complex, internal carotid artery, and labyrinth. Anterior and posterior petrosectomies aim to preserve the integrity of the labyrinth. A total petrosectomy will sacrifice the labyrinth; I believe this procedure is rarely, if ever, necessary.
In this chapter, I attempt to review the technical nuances for a standard anterior petrosal osteotomy and describe the operative maneuvers to minimize the need for additional bone removal when resecting lesions along the upper third of the clivus and ventrolateral midbrain and upper pons. The tenets for the middle fossa approach to vestibular schwannomas are discussed in another chapter.
Indications for the Anterior Petrosectomy
The most common indications for an anterior petrosectomy include:
- Intrapetrous lesions, with or without epidural extension, including:
- Anterior petrosal cysts (epidermoid or cholesteatomas)
- Chordomas and chondrosarcomas (endoscopic transnasal route preferred)
- Upper petroclival and Meckel’s cave tumors, including:
- Meningiomas
- Trigeminal schwannomas
- Intracisternal dermoid and epidermoid cysts extending across midline
- Lateral and ventral midbrain and pontine intra-axial lesions (cavernous malformations and tumors)
- Posterior circulation aneurysms (“low lying” basilar apex and basilar trunk aneurysms)
Some targets that are not suitable for this approach are:
- Purely clival tumors (originating medial to the groove of the inferior petrosal sinus or petroclival sulcus)
- Tumors extending caudal or originating caudal and/or posterior to the IAC
- Extensions of the tumor into the cranial nerves’ foramina
- Purely lower pontine and medullary lesions
As a general rule, the anterior petrosectomy should be preferred over conventional corridors for:
- Tumors entirely medial to and above the IAC
- Tumors that span the middle and posterior fossae
- Younger patients for whom more aggressive resection is desired
Figure 1: This right-sided upper petroclival/tentorial meningioma was removed via anterior petrosectomy (top photos). Note the extension of the tumor into the cavernous sinus; this small portion was not removed. The immediate postoperative scans are also included (bottom row).
Preoperative Considerations
Magnetic resonance (MR) imaging reveals the limits of the tumor and defines the candidacy of the tumor for this approach. Because of the limited working space offered via this route and its inflexibility for expansion intraoperatively, inappropriate patient selection leads to disappointing results and subtotal tumor removal. The need for combined operative corridors should be planned preoperatively.
A high-resolution CT scan of the temporal bone can provide additional information about the degree of pneumatization of the petrous apex. This imaging modality is especially beneficial for intraosseus lesions.
Some degree of temporal lobe retraction is required and the use of a lumbar drain is highly advised, especially for lesions on the dominant side. Intraoperative neurophysiologic monitoring of the facial nerve (electromyography) and brainstem auditory evoked responses (BAERs) is very helpful for localizing the facial nerve and warning the surgeon of maneuvers that may place the brainstem at risk.
Operative Anatomy
The ultimate goal of the anterior petrosal approach is to expose the ventral and ventrolateral aspects of the upper third of the brainstem. With this goal in mind, the importance of the steps involved in this skull base approach can be more readily rationalized. Tumors extending caudal to the IAC are not suitable for this approach, and a posterior petrosectomy should be considered to access them.
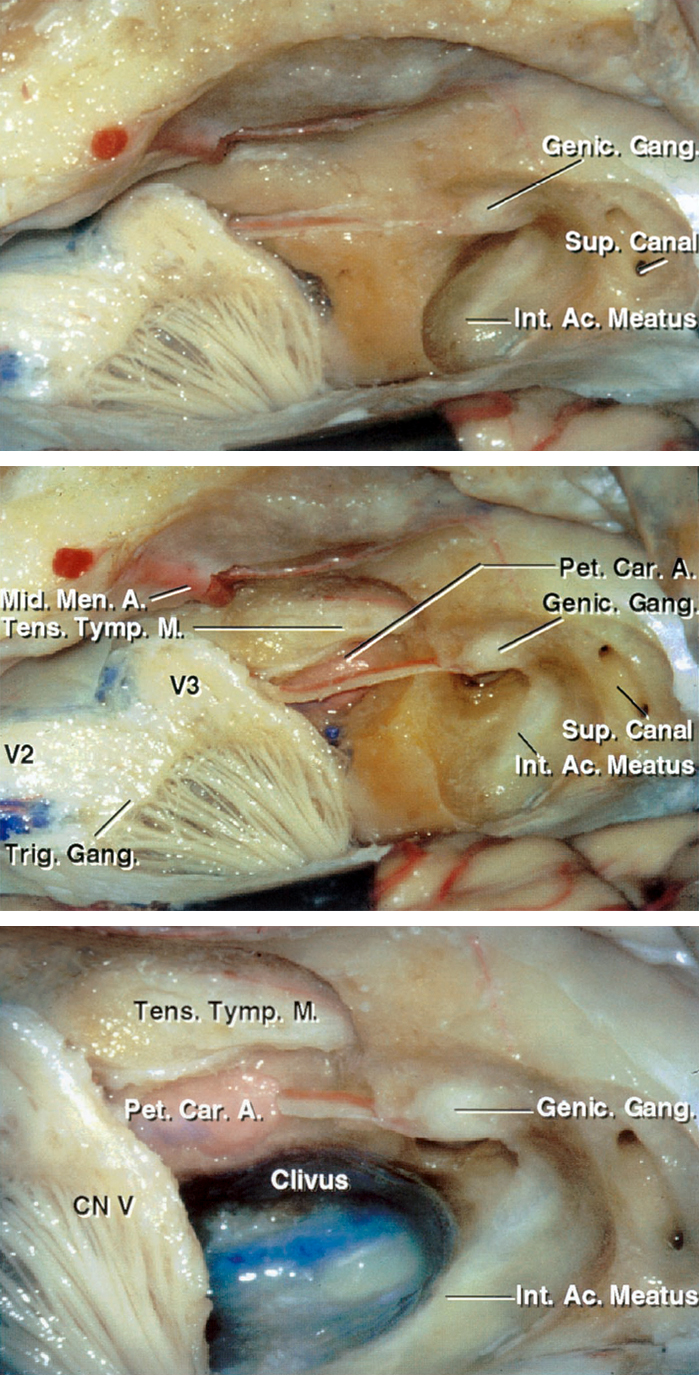
Figure 2: The bony anatomy of the middle fossa and petrous apex in relation to the surrounding tissues is demonstrated. Anterior petrosectomy involves removal of the bone housed in the middle fossa’s posteromedial triangle, also known as Kawase’s or quadrilateral triangle. This portion of the petrous apex is bordered anteriorly by the posterior margin of Meckel’s cave containing the mandibular division of the trigeminal nerve, posteriorly by the arcuate eminence, laterally by the greater superficial petrosal nerve (GSPN), and medially by the superior petrosal sinus (image courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 3: Stepwise resection of the petrous apex with skeletonization of the relevant surrounding anatomy is demonstrated. A close-up view of the right middle cranial fossa floor with the petrous apex and the bone over the IAC removed (lower image). Note the internal carotid artery along the anterolateral border of the bony resection. The inferomedial extent of the osteotomy is ultimately bounded by the clivus. The greater and lesser superficial petrosal nerves are parallel to the petrous carotid artery (images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 4: Intradural exposure after an anterior petrosectomy is shown. Note the location of the basilar artery and the extent of access to the anterolateral pons (image courtesy of AL Rhoton, Jr). The bony roof of the ICA is usually left intact and not removed. Deformation of these vital structures by the lesion alters the anatomy and the available working space after lesional decompression. These vital structures are often identified during the later stages of the operation and not easily exposed early.
ANTERIOR PETROSECTOMY (ANTERIOR PETROSAL APPROACH)
Before positioning the patient, I place a lumbar drain. Cerebrospinal fluid (CSF) drainage through the lumbar drain is imperative in avoiding temporal lobe injury during the lobe’s extradural retraction. Often 40 to 60cc of CSF is drained gradually (in 10cc aliquots) until the lobe is completely mobilized for adequate exposure of the middle fossa floor. I also prepare the lower quadrant of the patient's abdomen for harvesting fat grafts used for dural closure.
Patient Positioning
The patient is positioned supine on the operating table. A shoulder roll is placed under the patient's contralateral shoulder and the head rotated until the sagittal suture is parallel to or minimally angled from the floor. If the patient has a supple neck, he or she can tolerate up to 70 degrees of rotation. If the patient has a more rigid neck, the size of the shoulder roll can be increased to compensate for the lack of rotation.
For obese patients with less mobile necks, the lateral position must be used to avoid interruption of adequate venous return. I have a low threshold for using the lateral position because petrosectomy operations are often prolonged; physiologically neutral body positions (including neck posture) are associated with less discomfort after surgery.
Figure 5: Patient’s head position with the zygoma as the highest point on the operative field. The single pin of the skull clamp often has to be placed on the patient’s forehead to avoid the interference of the pins with the incision. The vertex of the patient's head is tilted slightly toward the floor. This maneuver maximizes the effect of gravity retraction on the temporal lobe.
The surgeon positions the skull clamp so the pins stay out of the operative working zone. My preference is to place the single pin anteriorly over the frontal area and the other two pins low on the occipital bone. A line connecting the single pin with the midpoint between the opposite two pins (swivel rocker arm) must always cross the equator of the patient's head to prevent skull clamp fixation failure during surgery.
Skin Incision
Numerous styles of skin incisions have been described for this approach.
Figure 6: The linear incision, as illustrated here, is adequate and heals most effectively. This incision starts near the inferior edge of the zygomatic arch, just in front of the superficial temporal artery that can be palpated during planning the incision. It extends cranially toward the vertex just above the superior temporal line. The linear incision mobilizes enough scalp and temporal muscle flaps to provide adequate skull exposure and obviates the need for more extensive incisions. Upon completion of the incision, the scalp is undermined and the attachments of the temporalis muscle to the superior temporal line are disconnected beyond the incision. This maneuver significantly expands the bony exposure through the linear incision.
The horseshoe incision (blue outline) is an alternative option for larger tumors. It starts immediately posterior to the ear and finally descends to the level of the zygomatic arch, about 1 to 2 centimeters in front of the tragus. The curvilinear incision (green outline) is another alternative that mobilizes the muscle effectively away from the operative working zone.
For a nonlinear incision, a myocutaneous flap is mobilized in a single layer. A myofascial cuff may be left on the bone to facilitate closure. For the horseshoe incision, the flap is reflected inferiorly with fishhooks; enough muscle dissection should be completed until the posterior root of the zygomatic arch is clearly visible. The operator must take care not to dissect so low as to violate the mandibular joint or external auditory canal.
Figure 7: Craniotomy through a horseshoe incision for a large tumor is illustrated. A single burr hole is placed as low as possible on the temporal squama, posterior to the root of the zygoma. The dura is generously stripped away from the inner surface of the calvarium. Lumbar CSF drainage can assist with dural mobilization. Next, the footplate of the craniotome elevates the bone flap with its inferior edge parallel and as close as possible to the floor of the middle fossa. The zygomatic arch defines the level of the middle fossa floor and may be used as a reference; this floor slopes slightly upward from anterior to posterior direction.
More burr holes may be used, if necessary, to preserve the integrity of the dura. Alternatively, the entire bony cut may be done using a diamond drill. Preservation of the dura’s integrity is crucial to avoid injury to the lobe during the later stages of extradural dissection along the middle fossa floor.
Figure 8: Craniotomy through a linear incision is illustrated. In this case, the fibers of the temporalis muscle are split longitudinally and the muscle attachments along the superior temporal line disconnected. When compared with the horseshoe incision, the posterior extent of exposure may be slightly compromised. Regardless which incision is used, the width of the craniotomy is two-thirds anterior and one-third posterior to the IAC. The root of the zygoma is exposed (arrow).
Figure 9: After the craniotomy flap is elevated, the remaining base of the temporal squama is reduced flush with the floor of the middle fossa. This goal can be accomplished using rongeurs or a drill with a side-cutting burr. Air cells are often exposed at this step of the operation and must be thoroughly obliterated with bone wax to prevent creation of a CSF fistula. The superior edge of the zygomatic arch may also be drilled to provide an unobstructed view of the floor of the middle fossa, but this is rarely necessary.
Figure 10: Additional removal of CSF through the lumbar drain relaxes the brain in preparation for its mobilization. Once the floor of the middle fossa is visible, I use a #1 Penfield dissector to detach the dura from the lateral aspect of the petrous bone. A self-retaining retractor may be placed on the dura to protect it during drilling. The remaining bone of the squama and bony gyrations along the lateral middle fossa floor are drilled completely flush with the lowest level of the middle fossa floor to improve the operative working angles while minimizing temporal lobe retraction.
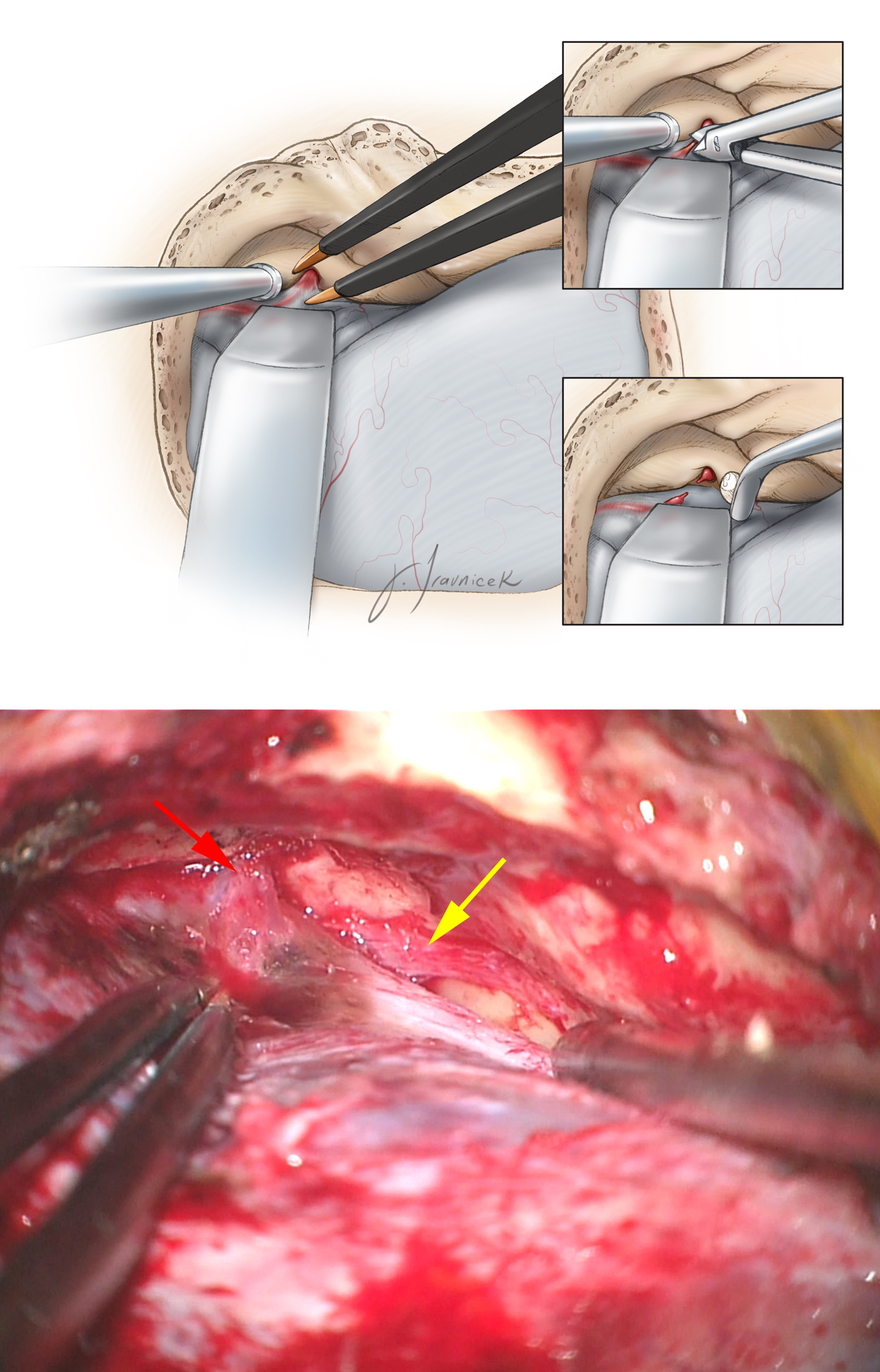
Figure 11: The dura over the floor of the anterior aspect of the middle fossa is now generously elevated. The middle meningeal artery (red arrow), entering through the foramen spinosum, is identified along the anterior aspect of the bone exposure. The artery must be coagulated on its dural side and transected. Next, I pack the foramen with bone wax and oxidized cellulose. Stripping of the dura anteriorly must stop at this point to avoid direct injury to the greater (GSPN)(yellow arrow) and lesser superficial petrosal nerves (LSPN), leading potentially to dry eyes. Inadvertent traction on the GSPN will also place the facial nerve at risk. The surgeon can minimize the risk of this type of injury by lifting the dura in a posterior-to-anterior direction.
The origin of the GSPN can be identified posteriorly at the hiatus; the dura is then stripped anteriorly in line with the nerve to prevent dislocation of the nerve out of its groove. The second caveat to the epidural exposure is to find the “true edge” of the petrous ridge. The superior petrosal sinus forms the petrosal groove along the superior aspect of the petrous ridge. The upper edge of this groove is often mistaken for the petrous ridge, resulting in inadequate elevation of the dura. Proper elevation of the dura along the ridge allows the retractor’s blade to readily rest against the petrous ridge and optimally retract the dura without slippage. I place two retractor blades: one along the arcuate eminence, and the second just lateral to the trigeminal impression.
Figure 12: Exposure of Kawase’s triangle (indicated by hash marks) is demonstrated. With the dura completely elevated to the level of the petrous ridge, the landmarks of Kawase’s quadrilateral are clearly visible: 1) laterally, the GSPN (and often the LSPN); 2) posteriorly, the arcuate eminence; 3) anteriorly, the posterior edge of the gasserian ganglion; and 4) medially, the petrous ridge.
The next step is to identify the meatal plane—the area of bone covering the IAC. Three ways of locating the meatal plane have been described. The Garcia-Ibanez technique relies on the relationship between the GSPN and arcuate eminence, usually located at an angle of 120 degrees. Bisection of this angle provides the general location of the IAC and a starting point for drilling the petrous ridge. Similarly, the Fisch technique uses an approximately 60-degree angle between the long axis of the arcuate eminence and the meatal plane to estimate the location of the IAC.
It is important to note that the anatomy of the middle fossa floor is widely variable, and the arcuate eminence is not always a reliable locator of the superior semicircular canal. As a general rule, the IAC courses at a 45-degree angle from a line that runs from the arcuate eminence perpendicular to the petrous ridge; this is my preferred method to locate the IAC.
In most cases of petroclival meningiomas, there is no need to fully expose the overlying dura of the IAC, and only the most medial aspect of the canal may be exposed as a reference. It is advantageous to dissect the dura propria from the sheath of V3 and the lateral border of the gasserian ganglion to optimize elevation of the temporal dura.
Figure 13: The bone within the boundaries of the Kawase’s quadrilateral region, anterior to the meatal plane, is carefully drilled using a rough diamond burr along with abundant irrigation to prevent thermal damage to the underlying structures. In most cases, the bone resection is kept posterior to the internal carotid artery (ICA) and anterior to the IAC unless there is a specific reason to expose these structures due to the particular features of the pathology at hand. The bone over the roof of the ICA may be missing or significantly attenuated, leading to early exposure of the artery; this fact should be kept in mind to avoid injury to the ICA. Also, to prevent damage to the cochlea, I abstain from drilling lateral to the ICA-IAC angle. The basic technique is to core out the central portion of the bony apex and later, using curettes, remove the thin shell of bone from the dura.
I usually ask my otology colleagues to be involved during the resection of the petrous apex.
Figure 14: All of the bone between the V3 anteriorly, the ICA and the cochlea laterally, and the superior semicircular canal and the IAC posteriorly is drilled away until the dura of the posterior fossa is reached, thereby completing the anterior transpetrosal osteotomy. A monopolar stimulating probe may be used for transdural mapping of the facial nerve. The bone along the margins of the cochlea is very compact and distinguishable from the cancellous bone of the petrous apex. Moreover, resection of bone can proceed beneath the trigeminal nerve as far as the lateral aspect of the clivus (*); however, the operator must take great care to prevent injury to cranial nerve V. Also, copious irrigation is necessary to prevent thermal injury to cranial nerve VI in the Dorello’s canal while drilling the medial extent of exposure. The operative corridor can be even further extended caudally by following the posterior fossa dura as far as the inferior petrosal sinus. The route of the carotid artery is marked with the red arrow. The posterior fossa dura is at the tip of the suction device.
INTRADURAL PROCEDURE
Dural incisions should maximize the intradural extent of exposure.
Figure 15: The dural opening is planned as a T-shaped incision. The first incision is made along the basal temporal dura parallel to the inferior edge of the craniotomy. The second cut is perpendicular to the first, crossing the superior petrosal sinus into the posterior fossa dura. This dura is often infiltrated by the tumor/meningioma and may be resected. I often make two separate dural incisions separated by the superior petrosal sinus. The sinus is then ligated using a Weck clip and the dural incisions connected. Note the location of the trigeminal nerve within the dura; this nerve can be easily and inadvertently transected if not properly located (please see more details below).
Figure 16: The posterior limb of the incision must be carefully completed to ensure that the vein of Labbé is not injured. I advance the retractors intradurally to protect the brain during the deeper dural incisions. The vein of Labbé may be followed to its insertion point to ensure that its outflow is not obliterated during the division of the superior petrosal sinus. The tentorium is then transected posteriorly to the point where cranial nerve V enters the dura. The operator should also be especially careful during these dural incisions to protect the trigeminal nerve—it can easily be inadvertently transected due to its displacement by the tumor as it enters the Meckel cave at the porus trigeminus.
Figure 17: The end result of the procedure is a generous ventrolateral view of the structures of the posterior fossa through a middle fossa corridor. A ventral pontine cavernous malformation can be readily resected through this route. The underlying extra-axial pathology affects the pattern of displacement of the regional neurovascular structures (upper sketch). Cranial V is the center of the operative corridor in this approach and can be easily injured during dural opening and tumor resection (middle image). The final outcome of resection is noted in the lower image; a small amount of tumor infiltrating the cavernous sinus is left behind (arrow).
Closure
Primary dural closure is obviously impossible in this area and alternative methods are needed. Adipose tissue with its globular texture is one of the best barriers against CSF leakage. Strips of adipose tissue are placed across the dural opening to seal the dural defect. Before placement of the adipose grafts, all air cells including the ones at the petrous apex and mastoid area must be meticulously waxed.
Alternatively, a vascularized muscle flap prepared from the posterior aspect of the temporalis muscle may be rotated to fill the defect in the dura. This latter method is used during repeat operations for patients who have undergone radiation treatment. The bone flap is replaced and secured using miniplates, and the rest of closure is conducted in the standard fashion.
A lumbar drain is used to drain 8cc per hour of CSF for 48 hours after surgery. A head CT scan the morning after surgery should exclude significant pneumocephalus before the drain is utilized. Patients are mobilized as soon as possible.
If postoperative rhinorrhea is encountered, a lumbar drain is reinstituted and continued for 3-4 days. If leakage is evident after discontinuation of the lumbar drain, repeat operative intervention with rewaxing of the apical air cells and repacking of the resection cavity with fat strips is required. The lumbar drain is then continued for another 2 to 3 days.
Pearls and Pitfalls
- Patient selection is one of the key elements for operative success. Anterior petrosectomy is designed to access the ventral and ventrolateral regions of the upper brainstem or the upper third of the clivus. This corridor is not designed to reach below the IAC. This approach offers a narrow, inflexible working zone and does not allow the operator to reach large tumors. Large tumors may require a staged approach through adjuvant routes.
- A lumbar drain is indispensable to avoid retraction injury. The dura must remain intact until the operator intentionally opens the dura.
- If lumbar CSF drainage does not allow adequate temporal lobe decompression, the procedure should be abandoned and an alternative approach contemplated at a different operative session. Forceful lobar retraction is hazardous and leads to disappointing results.
- Patience is important during petrous bone drilling. Practice in the laboratory is an indispensable prerequisite for safety and efficacy of petrosectomy. Our ENT colleagues are essential members of our team during this procedure.
- Incisions within the dura must be carefully placed. Tumors displace cranial nerves and vessels, and injury to these vital structures during the dural opening is often most unexpected. It is OK to say “there it is” and be wrong a thousand times, but it is not OK to say “there it was” and be right even once.
For additional illustrations of the middle fossa-transpetrous apex approach to the anterosuperior cerebellopontine angle, please refer to the Jackler Atlas by clicking on the image below:
References
Al-Mefty O. Operative Atlas of Meningiomas. Philadelphia: Lippincott-Raven, 1998.
Aziz KM, van Loveren HR, Tew JM Jr, Chicoine MR. The Kawase approach to retrosellar and upper clival basilar aneurysms. Neurosurgery. 1999;44:1225-1234; discussion 1234-1236.
Cappabianca P, Califano L, Laconetta G (eds). Cranial, Craniofacial and Skull Base Surgery. Milano: Springer-Verlag Italia, 2010.
Diaz Day J. The middle fossa approach and extended middle fossa approach: technique and operative nuances. Neurosurgery. 2012;70 (2 Suppl Operative):192-201.
Gonzales LF, Lekovic GP, Kakarla LK, Reis CVC, Weisskopf P, Daspit CP. Surgical approaches to the cerebellopontine angle, in Surgery of the Cerebellopontine Angle. Bambakidis NC, Megerian CA, Spetzler RF (eds). Shelton, CT: People’s Medical Publishing House, BC Decker, 2009, chapter 5
Miller CG, van Loveren HR, Keller JT, Pensak M, el-Kalliny M, Tew JM Jr. Transpetrosal approach: surgical anatomy and technique. Neurosurgery. 1993;33:461-469; discussion 469
Roche PH, Lubrano VF, Noudel R. How I do it: epidural anterior petrosectomy. Acta Neurochir (Wien). 2011;153:1161-1167.
Tew JM Jr, van Loveren HR. Atlas of Operative Microneurosurgery, Vol 1. Philadelphia: Saunders, 1994.
Tew JM Jr, van Loveren HR, Keller JT. Atlas of Operative Microneurosurgery, Vol 2. Philadelphia: Saunders, 2001.
Please login to post a comment.