Epidermoid and Dermoid Cysts
This is a preview. Check to see if you have access to the full video. Check access
Resection of a Multicompartment Cerebellopontine Angle/Petroclival Epidermoid Tumor via the Retrosigmoid Transtentorial Approach
Epidermoid and dermoid tumors are actually not neoplasms, but rather ectoderm-lined inclusion cysts. These lesions are thought to arise during neural development when surface ectoderm does not entirely separate from the neural tube between the third and fifth weeks of embryonic development. They can also be iatrogenic after lumbar puncture or traumatic following stab injuries.
Epidermoids are composed strictly of squamous epithelium, whereas dermoids contain elements of all layers of skin, including squamous epithelium, hair, sebaceous glands, and fat. Dermoids and epidermoids account for about 1% of all intracranial tumors, are very slow growing, and have been associated with craniovertebral junction anomalies and Klippel-Feil syndrome.
Malignant transformation into squamous cell carcinoma is possible, but very rare. Epidermoids are most commonly found in the cerebellopontine (CP) angle and are the third most common tumor in that location after vestibular schwannomas and meningiomas. They also occur in the parasellar region and grow lineally, similar to normal skin. Dermoids are more often found in the midline and may have a sinus tract connecting to the skin, which may place the patient at risk for recurrent meningitis. These tumors may also cause focal changes at the skin surface, such as a tuft of hair, altered skin pigmentation, or spina bifida.
Clinical Presentation
Since epidermoid and dermoid tumors commonly occur in the CP angle, facial weakness and unilateral hearing loss are the most common presenting symptoms. However, headaches, facial pain, facial spasms, and ataxia have also been commonly reported based on the size of the lesion.
Dermoids are typically midline, so they may present with similar symptoms as sellar, parasellar, or pineal tumors, such as visual field or other cranial nerve deficits, pituitary dysfunction, or obstructive hydrocephalus. Rarely, these lesions may spontaneously rupture, causing severe chemical meningitis during which patients present with severe headache, photophobia, neck stiffness, and nausea/vomiting.
Evaluation
Special attention is paid to the function of cranial nerves, brainstem, and cerebellum as well as stigmata of pituitary hormone dysfunction. Epidermoids are typically hypodense and appear cystic on computed tomography (CT) scan without contrast enhancement or associated edema. The differential diagnosis includes arachnoid cyst, Rathke's cleft cyst, craniopharyngioma, and other cystic tumors, which are very problematic to distinguish. Evaluation of Hounsfield units may show the mass to have more fat density than cerebrospinal fluid (CSF) density.
Magnetic resonance (MR) imaging shows hypointense lesions on T1 and hyperintense on T2-weighted sequences with minimal contrast enhancement. This imaging pattern often causes the interpreter to confuse epidermoids with arachnoid cysts; however, diffusion-weighted imaging (DWI) sequences easily distinguish these pathologies as epidermoids because they are hyperintense on DWI, whereas arachnoid cysts are not. Dermoids are hyperintense on T1-weighted sequences because of their high fat content.
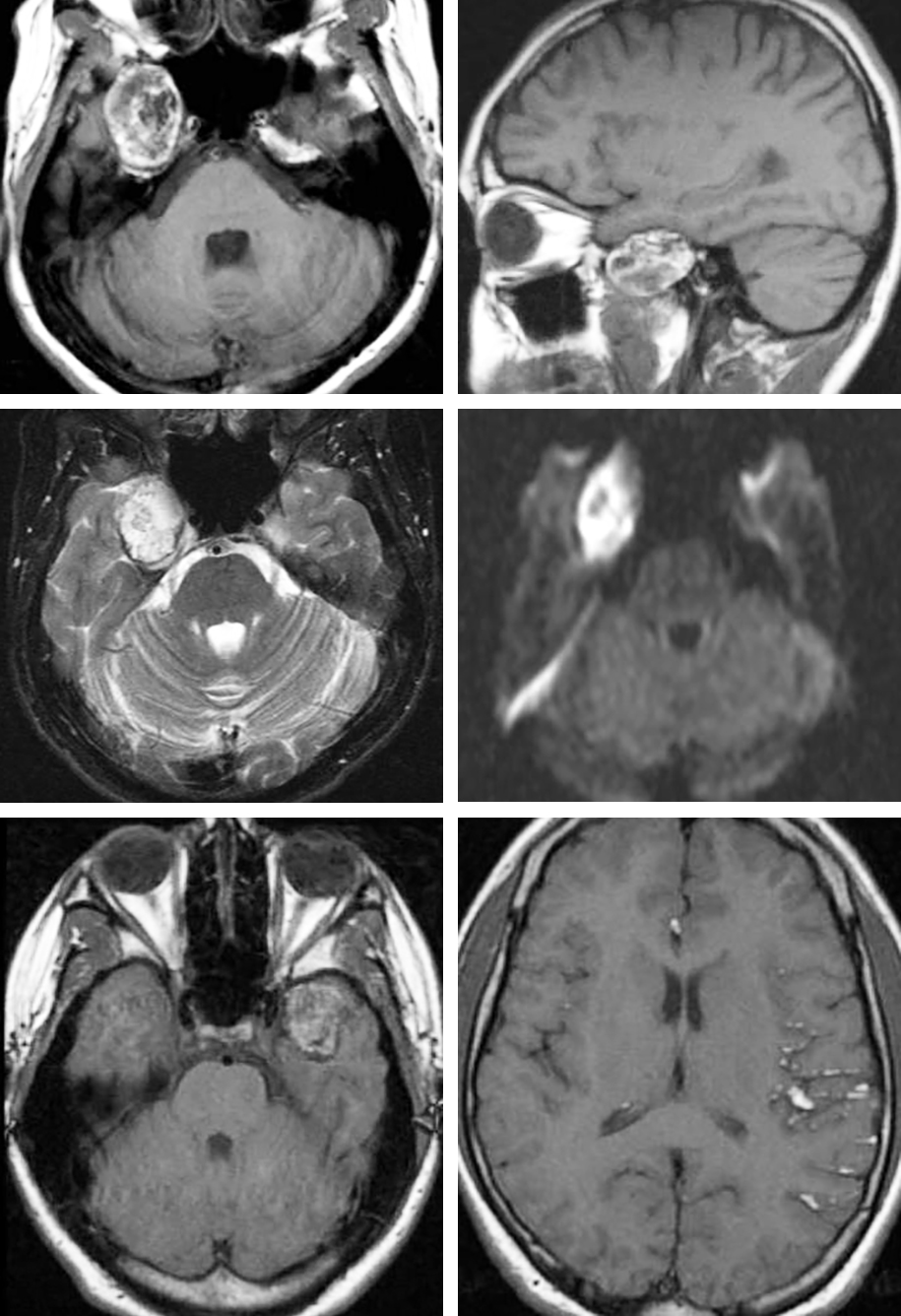
Figure 1: This CP angle tumor demonstrates the T2 hyperintensity characteristic of CSF and DWI hyperintensity, nearly pathognomonic of epidermoid tumors.
Figure 2: This right-sided temporal dermoid lesion demonstrates T1 hyperintensity (images in upper row) consistent with its fat content. The appearance of T2- and diffusion-weighted sequences are shown in the images of the middle row. Since the texture of dermoids is more solid than epidermoids, dermoids are less likely to grow around neurovascular structures and are more likely to cause focal mass effect. Some dermoid tumors may show nodular enhancement. The pattern of a left temporal dermoid rupture leading to disseminated subarachnoid and intraventricular fat droplets is shown in the images of the bottom row.
Preoperative Considerations and Planning
Neurophysiological monitoring including brainstem auditory evoked responses (BAERs) is recommended for removal of CP angle lesions. I do not use facial nerve monitoring for these tumors because the adherent capsule should be left on the cranial nerves; removal of this part of the capsule should not be attempted. I favor the use of a lumbar drain for early posterior fossa decompression as the large tumors can fill the CP angle cisterns and prevent adequate CSF release upon cisternal opening.
Microsurgical resection is the only reliable treatment for epidermoids and dermoids. The goal of surgery is maximal safe resection while preserving function. Ideally, the entire tumor and capsule are removed to minimize the risk of recurrence; however, the capsule is densely adherent to surrounding neurovascular structures and therefore defies its complete, safe microsurgical excision. Parts of the capsule that are adherent to the surrounding cranial nerves and vessels should be left behind to prevent morbidity.
The epidermoid tumors are well demarcated and own a smooth, hypovascular capsule. The cyst contains characteristic pearly flakes. Epidermoid cysts contain epithelial cell debris and keratin, whereas dermoids contain elements of the dermis, including hair and hair follicles, as well as apocrine, sebaceous, or sweat glands.
The risk of chemical meningitis associated with intraoperative spillage of cyst contents or preoperative cyst rupture is significant, and the resultant radiographic findings of small fat globules in the subarachnoid and intraventricular spaces are characteristic features of dermoids. Steroid administration relieves the symptoms of meningitis; however, the ensuing hydrocephalus may require definitive CSF shunting.
There is no evidence to support the role of radiotherapy or chemotherapy in the treatment of residual or recurrent dermoids and epidermoids.
Operative Anatomy
The details of posterior fossa operative anatomy are reviewed elsewhere in this atlas including in the Retromastoid Craniotomy chapter. Both dermoid and epidermoid tumors most commonly occur within the midline posterior fossa and CP angle, respectively.
Click here to view the interactive module and related content for this image.
Figure 3: Cranial nerves of the CP angle through a right-sided retrosigmoid approach are shown. The vestibulocochlear nerve enters the IAC along with the labyrinthine branch of the anterior inferior cerebellar artery (AICA). Protection of this artery is imperative for hearing preservation (images courtesy of AL Rhoton, Jr).
Surgical Approach
Epidermoids are more common than dermoids and typically occur within the CP angle cisterns. Therefore, the following discussion of operative techniques relates to resection of CP angle epidermoid cysts.
The retrosigmoid approach is a versatile route for removal of CP angle epidermoid cysts. Because resection of this tumor type is much more likely to improve the patient’s hearing than resection of acoustic neuromas, the hearing status is not a consideration in selection of the surgical approach; the translabyrinthine approach sacrifices hearing and is avoided.
For extensive multicompartment lesions, the transpetrosal approach or other skull base corridors may be required. In my experience, even giant multicompartment CP angle epidermoids that cross the ventral midline can be resected through the extended retrosigmoid craniotomy. The pearly flakes of this tumor are readily removed using the suction apparatus and via limited space for microsurgery. Strategic exposure of the deep spaces via interneural working channels using the dynamic retraction methodology is effective for reaching ventral brainstem.
RESECTION OF CEREBELLOPONTINE ANGLE EPIDERMOID CYSTS
Please refer to the Extended Retrosigmoid Craniotomy chapter for description of the craniotomy and exposure. Note that larger tumors require special modifications of the retrosigmoid route. These modifications are described below.
The use of radical and complex skull base osteotomies, including petrosal approaches, is unlikely to significantly add to the safety and extent of lesion removal for these specific tumors. The nonfibrous texture of epidermoid and dermoid lesions facilitates their excision via narrow operative corridors. The extent of resection is limited by the adherence of the tumor capsule to contiguous neurovascular structures rather than the breadth or span of the exposure.
The patient is positioned in the lateral or park-bench position. The patient’s ipsilateral shoulder is pulled forward and toward his or her foot and secured in place with tape; this prevents interference of the shoulder with the operative space around the retromastoid region. A bulky shoulder significantly limits the surgeon’s ability to work within the retromastoid region, and appropriate preparations should be made to avoid this obstacle. If the patient is morbidly obese, the sitting position is a more appropriate choice.
Figure 4: Patient positioning for a retrosigmoid removal of a large epidermoid cyst is illustrated. The head is slightly turned toward the floor and tilted down to expand the space between the patient’s shoulder and the suboccipital region. To further broaden this working space, I gently mobilize the shoulder anteriorly and inferiorly (green arrow) and tape it in place. A lumbar puncture is performed and 30 to 40 cc of CSF is removed or a lumbar drain is placed.
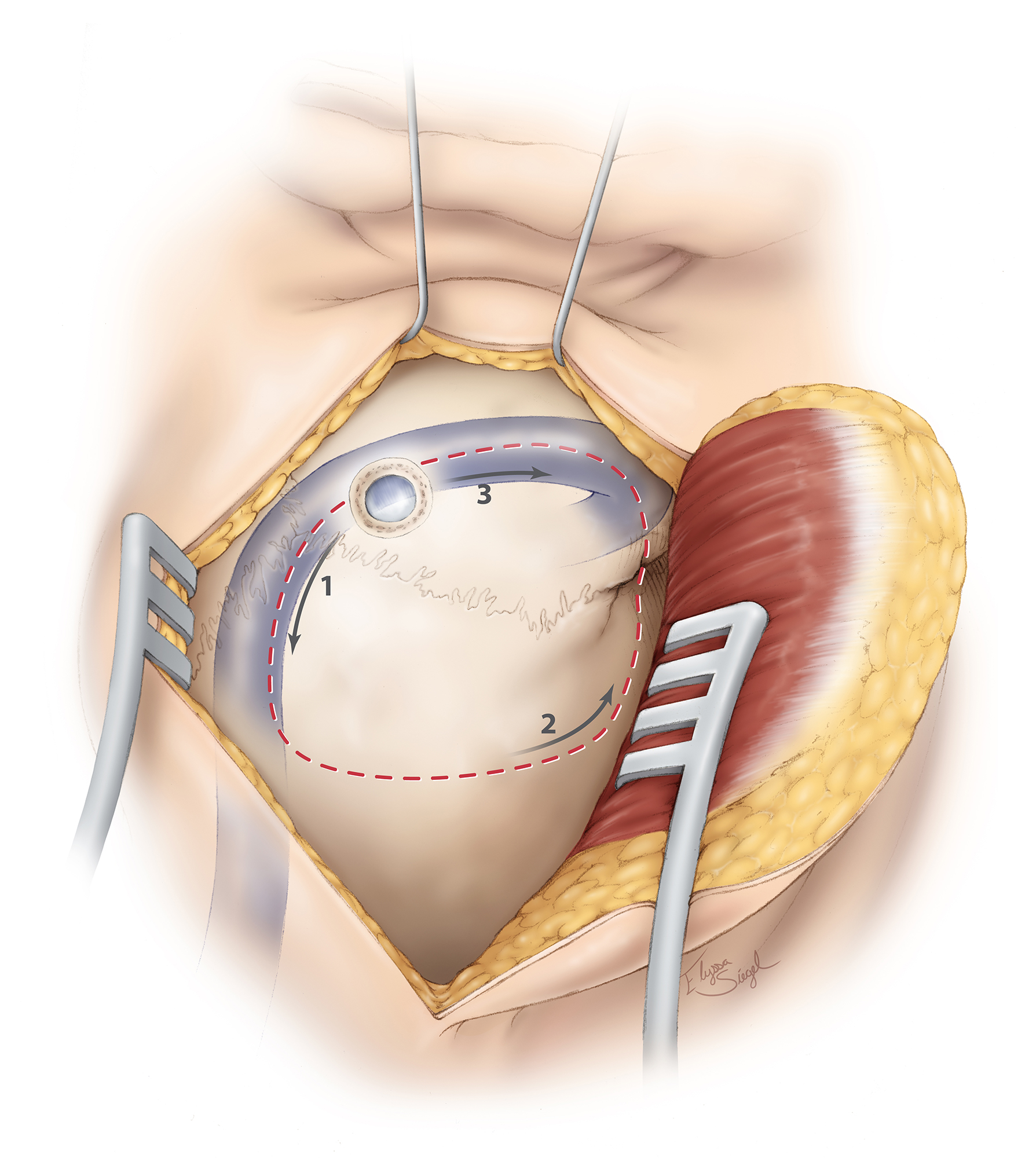
Figure 5: I approximate the junction of the transverse and sigmoid sinuses by first localizing the transverse sinus, estimated by a line extending from the root of the zygoma to the inion. The junction of this line and the vertical line through the mastoid groove is a relatively good estimate of the location of the transverse-sigmoid junction. A curvilinear skin incision is then planned, with its summit just above the junction as illustrated. The base of the incision is wide according to the size of the tumor. Intraoperative image guidance can also assist with identification of the dural venous sinuses and planning of the incision.
Figure 6: A burr hole is made just at the edge or over the junction of the dural venous sinuses. The dura is carefully stripped from the inner table of the suboccipital bone, and a wide retrosigmoid craniotomy bone flap is elevated using the craniotome. The last bone cut is completed just posterior to the sigmoid sinus that is embedded in the skull and can be unroofed as a second step to avoid its injury. The bone over the floor of the posterior fossa is not removed. CSF drainage through the lumbar drain assists with dural relaxation and mobilization of the venous sinuses away from the drill’s footplate.
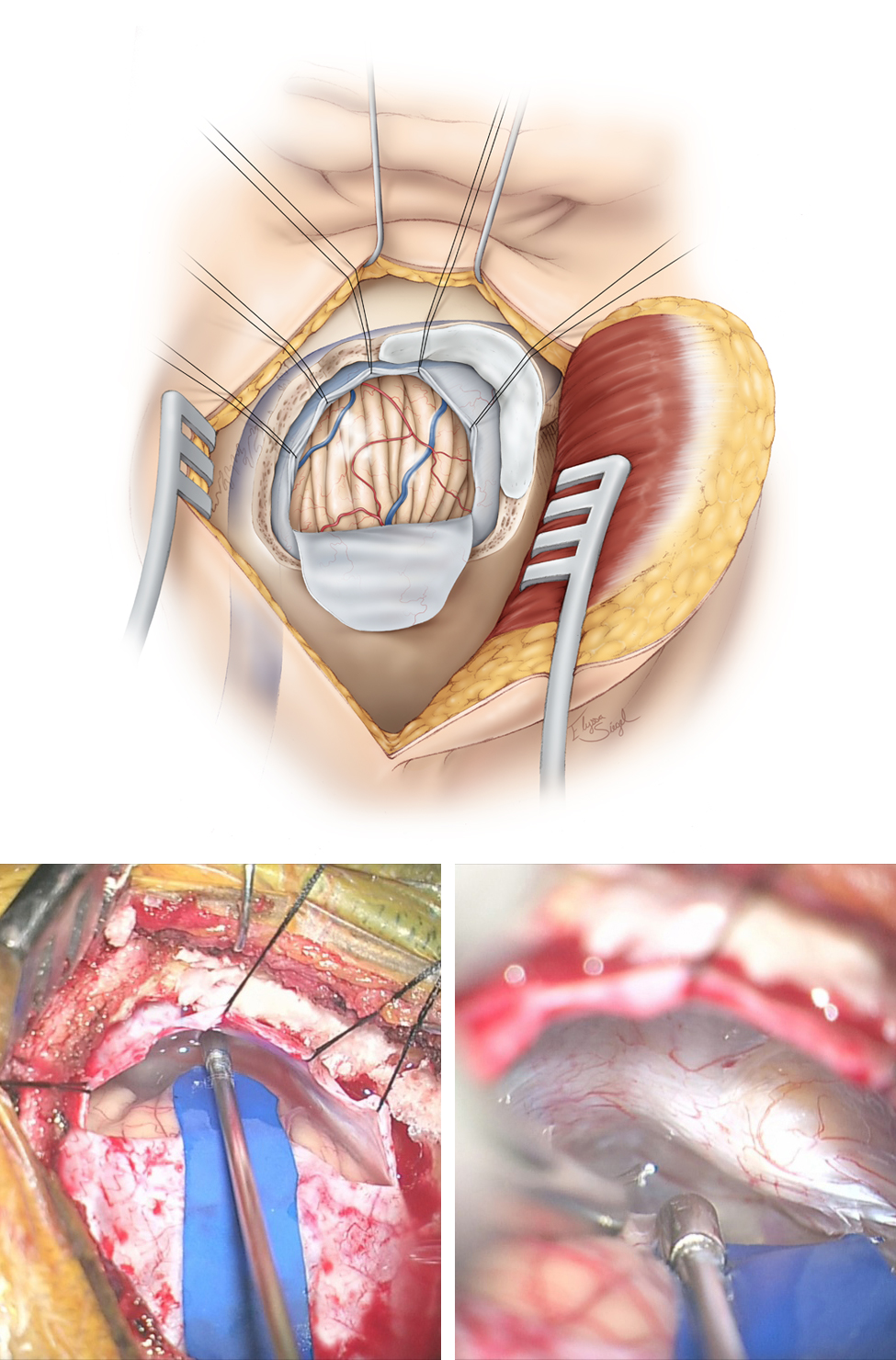
Figure 7: Please note the use of fishhooks and a cerebellar retractor to effectively mobilize the scalp and expand the bone exposure. Additional bone over the transverse and sigmoid sinuses is removed using an air drill. Bone removal over the sigmoid sinus is important for its mobilization. All mastoid air cells are generously waxed. A dural incision is then made parallel and adjacent to the transverse and sigmoid sinuses (upper sketch). This style of dural opening prevents dessication of the dural flap from the heat of the microscope. The dural edges along the venous sinuses are tented up using retraction sutures to widen the operative corridor toward the CP angle by mobilizing the sigmoid sinus laterally, decreasing the need for cerebellar retraction. Further lumbar CSF drianage avoids cerebellar herniation through the craniotomy defect. The petrous-tentorial junction is pursued; a piece of rubber glove (“rubber dam”) is used to allow the instruments to glide over the cerebellum (lower row of images).
INTRADURAL PROCEDURE
If necessary, the hemisphere is gently elevated and CSF is drained from the cisterna magna. A lumbar drain obviates the need for this maneuver.
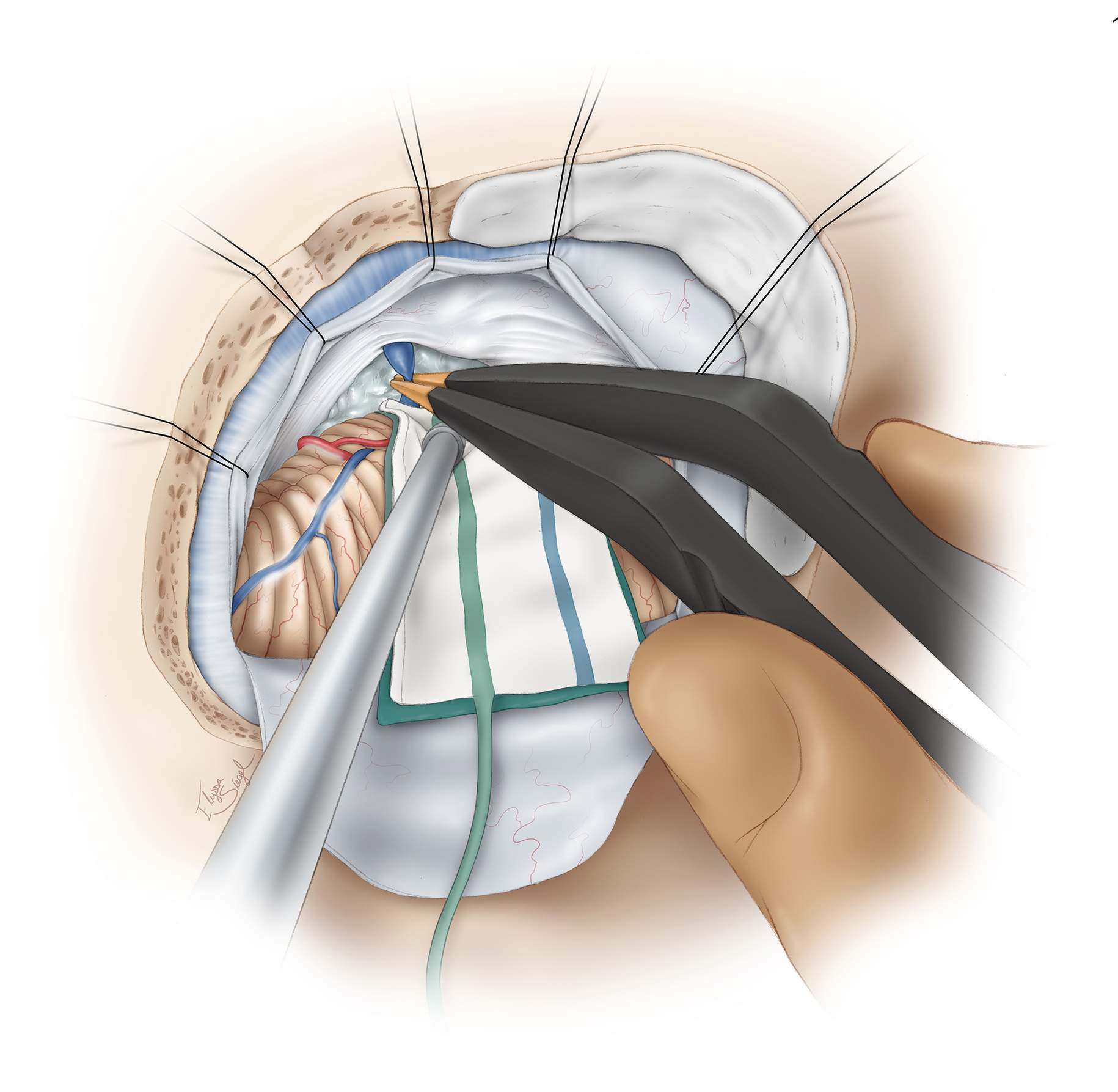
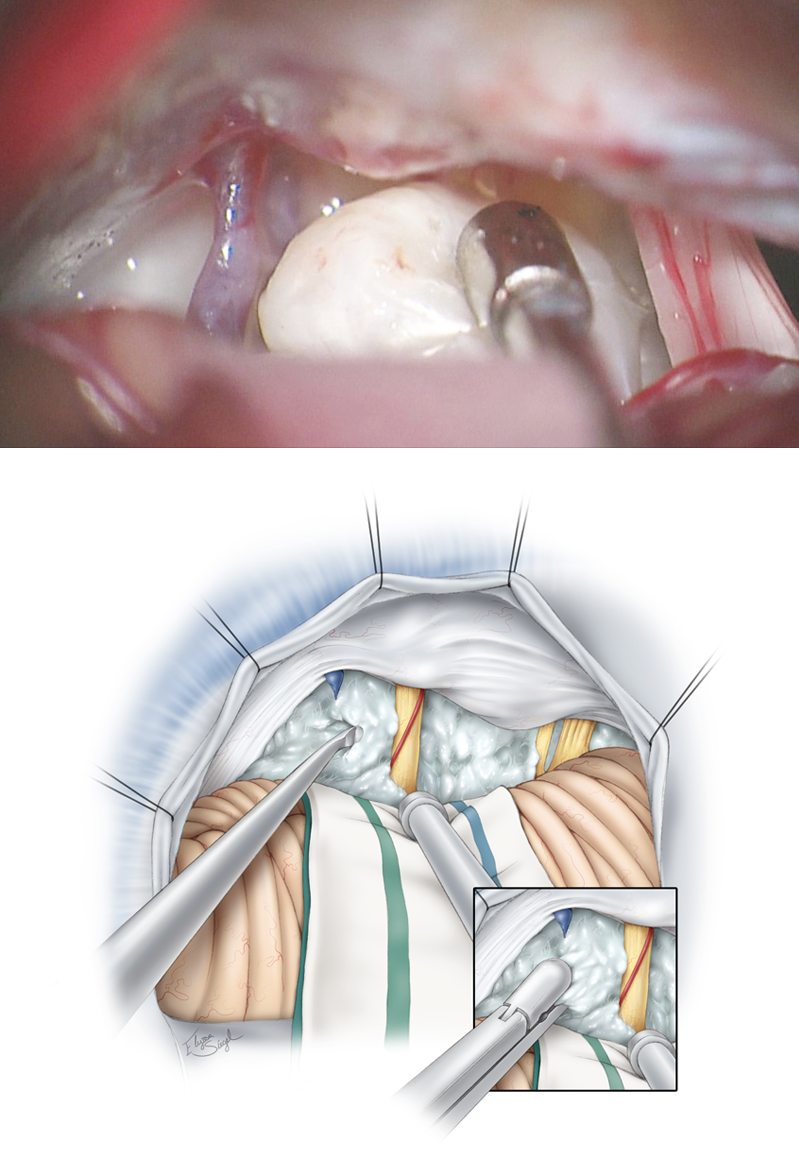
Figure 8: Medial mobilization of the petrosal surface of the hemisphere allows exposure of the tumor within the CP angle. The petrous-tentorial junction is a reliable landmark for anatomic orientation. The superior petrosal vein is coagulated and transected; this maneuver untethers the cerebellum and creates a wider operative space. The arachnoid bands over the cranial nerve (CN) VII/VIII complex are released to minimize traction on these nerves.
When anatomically possible, I attempt to find the neurovascular structures early in dissection to keep them out of harm’s way. Branches of the superior and anterior inferior cerebellar arteries, including the labyrinthine artery, CN V, and the CN VII/VIII complex, are identified, and tumor removal begins using the corridors found between the tentorium and CN V, between CN V and CNs VII/VIII, as well as between CNs VII/VIII and IX/X. CN V is most tolerant and CN VIII is least tolerant of manipulation. Monitoring BAERs is somewhat protective of CN VIII during operative manipulations.
CN V is typically displaced anteriorly and not visible until substantial tumor removal is accomplished. However, CNs VII/VIII are more superficial or anatomically posterior, and are therefore more likely to be mobilized further inferiorly and posteriorly by the mass. The center of the tumor is frequently just posterior and inferior to the root entry zone of CN V. The posterior tumor capsule is sharply incised between the tentorium and CNs VII/VIII and intracapsular decompression is conducted. The pearly flakes can be easily removed using the handheld suction device or pituitary rongeurs.
Figure 9: The anatomy of the upper CP angle with the superior petrosal vein and the CN VII/VIII complex bordering the tumor is indicated (upper photo). I “scoop” and morselize the tumor using a “cup” dissector, starting between the tentorium and around CNs VII/VIII. The flakes of the epidermoids are readily mobilized and disconnected. Following this defragmentation technique, I use the pituitary rongeurs to remove the disconnected tumor globules (bottom illustration, inset image). The mass of the flakes may engulf rather than displace neurovascular structures. The lesion should not be grabbed blindly with the pituitary rongeurs, fragmented and pulled out. Dermoid tumors can be removed in a similar fashion due to their high fat content and globular texture.
Figure 10: Tumor debulking proceeds from medial to lateral directions within the CP angle. I use an angled “pancake dissector” to mobilize the capsule (left upper image), and then use the pituitary rongeurs to remove the tumor flakes mobilized into the resection cavity (right upper image). Sharp dissection is used to excise the tumor capsule from the neighboring neurovascular structures (lower sketch). Although gentle removal of the capsule is attempted, aggressive handling of the cranial nerves is not advised. Encased perforating vessels are left untouched.
Figure 11: Once the tumor in the ventral space ipsilateral to the basilar artery has been removed, exploration of the tumor beyond and on the contralateral side of this artery begins. This maneuver should be done via dynamic retraction on the artery using the hand-held suction device. Blind manipulation of and traction on tissues can result in perforator injury. The corridor between CNs V and VII/VIII is ideal for exploring resection of the lesions crossing the midline. It is important to avoid direct traction on the CN VII/VIII complex; however, the tolerant CN V may be gently manipulated if necessary. The encasing arachnoid membranes of the tumor on the pia, contralateral to the basilar artery, are used to deliver the tumor into the operator’s view (inset image). Overall, the encasing arachnoid bands provide an effective “handle” to mobilize the tumor during various steps of lesion removal.
I usually find a pial location, most frequently just around the root entry zone of CN V, where the tumor demonstrates intimate adherence to the pia and, in fact, invasion into the brainstem parenchyma. I have always assumed this is the exact site where the surface ectoderm did not entirely separate from the neural tube between the third and fifth weeks of embryonic development, leading to formation of epidermoid lesions. Removal of this specific attachment point, which includes thickened flakes, requires minor invasion of the pia and parenchyma; however, this action is feasible around the root entry zone of CN V with minimal neurologic consequences.
Figure 12: Epidermoid lesions of the CP angle may adhere to the brainstem around the root entry zone of CN V. The top row images show the invasion of the pia at this location. The left middle image demonstrates the dissection of the lesion from the nerve. When the tumor invades the parenchyma over a significant area, a layer of the capsule is left behind (right middle image). The final operative field is indicated in the lower photo. The initial embryologic point of tumor attachment at the root entry zone of CN V is evident.
Figure 13: Epidermoid flakes are notorious for hiding within and around the arachnoid planes and in the cerebellopontine fissure. Careful endoscopic inspection of the blind operative spots and, more specifically, the axilla of the CNs, tentorial incisura, and mesecephalic/pontine cerebellar fissures, is highly recommended as I invariably find tumor fragments in these areas after microscopically-confirmed gross total removal. A final view of the resection cavity and the critical structures that should be preserved during microsurgery is indicated. Numerous rounds of irrigation fluid can also displace and remove fine flakes that have been overlooked and hidden within the arachnoid bands.
CP Angle Epidermoid: Dissecting Neurovascular Structures
Resection of a Large Recurrent Third Ventricular Epidermoid Tumor via the Posterior Interhemispheric Transcallosal Approach
Dermoid Lesions
Dermoid lesions are more focal, confined and do not tend to engulf neurovascular structures. Therefore, they are more amenable to gross total resection.
Figure 14: A fourth ventricular dermoid tumor in a patient with intractable headaches and mild hydrocephalus is shown. Note the subtle punctate T1 hyperdensities within the lesion, characteristic of fat droplets (top images). The lesion was accessed via the telovelar approach. The tumor contained hair (middle photo). Upon complete removal of the contents of the lesion’s capsule, the aqueduct was visible (bottom photo).
Fourth Ventricular Dermoid Cyst
Closure
Following tumor resection, the resection cavity is thoroughly irrigated and inspected, and hemostasis is achieved. A primary watertight dural closure is attempted. If this is not possible due to desiccation of the dura, a dural substitute may be used to complete a watertight closure. Patients who have undergone resection of their dermoid or epidermoid tumors are at risk of postoperative increased CSF pressures, leading to CSF leakage. Development of delayed hydrocephalus is not unlikely in these cases.
Bone wax is reapplied to the edges of the cranial defect to ensure obliteration of the mastoid air cells. Following replacement of the bone flap or cranioplasty, soft tissues are closed in the standard anatomic manner. The lumbar drain apparatus is removed at the completion of surgery.
Postoperative Considerations
Patients are admitted to the intensive care unit postoperatively. A common complication of epidermoid or dermoid resection is chemical meningitis. This complication manifests in a delayed fashion (within 1-2 weeks) as severe headaches with photophobia, neck stiffness, nausea and vomiting, and often back and leg pain. Nonspecific symptoms, including intractable headaches and nausea, may be the only manifestations. Cranial nerve deficits such as vertigo, hearing loss and facial weakness may occur. Bacterial meningitis should be ruled out. Administration of steroid medications is usually helpful in controlling these symptoms that often resolve within 1 to 2 weeks.
Pearls and Pitfalls
- Patients with epidermoid and dermoid cysts often present with posterior fossa syndrome. Epidermoid lesions should not be confused with arachnoid cysts. Microsurgical resection is their mainstay mode of therapy.
- Complex skull base approaches are not necessary for resection of these cysts. The retrosigmoid approach is adequate, even for ventral midline lesions abutting both sides of the basilar artery.
- The clinician should have a low threshold of suspicion for postoperative chemical meningitis after resection of these masses.
Contributor: Andrew R. Conger, MD, MS
References
Berger MS, Wilson CB. Epidermoid cysts of the posterior fossa. J Neurosurg. 1985;62:214-219
Chandra PS, Gupta A, Mishra NK, Mehta VS. Association of craniovertebral and upper cervical anomalies with dermoid and epidermoid cysts: report of four cases. Neurosurgery. 2005;56:E1155
Chu CK, Tseng HM, Yound YH. Clinical presentation of posterior fossa epidermoid cysts. Eur Arch Otorhinolaryngol. 2006;263:548-551
Cohen-Gadol A, Al-Mefty O. Skull base tumors, in Bernstein M, Berger MS (eds): Neuro-Oncology. New York: Thieme, 2008.
Kobata H, Kondo A, Iwasaki K. Cerebellopontine angle epidermoids presenting with cranial nerve hyperactive dysfunction: pathogenesis and long-term surgical results in 30 patients. Neurosurgery. 2002;50:276-285
Link MJ, Cohen PL, Breneman JC, Tew JM Jr. Malignant squamous degeneration of a cerebellopontine angle epidermoid tumor. Case report. J Nerousurg. 2002;97:1237-1243
Samii M, Draf W, Lang J. Surgery of the Skull Base: an Interdisciplinary Approach. Berlin: Springer; 1989
Samii M, Sam SA, Herold C, Samii A. Congenital rest lesions and rare tumors, in Bambakidis N, Megerian C, Spetzler R (eds): Surgery of the Cerebellopontine Angle. Shelton, CT: People’s Medical Publishing House, 2009.
Samii M, Tatagiba M, Piquer J, Carvalho GA. Surgical treatment of epidermoid cysts of the cerebellopontine angle. J Neurosurg. 1996;84:14-19
Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437-1455
Tamura K, Aoyagi M, Wakimoto H, et al. Malignant transformation eight years after removal of a benign epidermoid cyst: a case report. J Neurooncol. 2006;21:419-438
Yasargil MG, Abernathey CD, Sarioglu AC. Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery. 1989;24:561-567
Please login to post a comment.























Comments: