Tuberculum Sella Meningioma
This is a preview. Check to see if you have access to the full video. Check access
Endoscopic Transnasal Microsurgical Resection of Tuberculum Sella Meningioma
Representing 5 to 10% of intracranial meningiomas, tuberculum and diaphragm sellae meningiomas are intimately involved with the critical suprasellar structures: the optic nerves and chiasm, the hypothalamic-pituitary axis, and the internal carotid-anterior cerebral artery complex. Unlike their counterparts that arise more laterally from the clinoid process or sphenoid wing, these suprasellar lesions are potentially resectable through an expanded endonasal approach.
In appropriately selected patients and when compared with the transcranial route, the endonasal approach decreases the risk of iatrogenic injury to the optic apparatus and allows early tumor devascularization. For transcranial methods of resection, refer to the chapter on Tuberculum Sella Meningioma in the Cranial Base Surgery volume.
Diagnosis
Because of their intimate and early involvement with the optic chiasm, patients with a tuberculum sellae meningioma nearly always present with signs of optic apparatus dysfunction. Although a bitemporal hemianopsia is the classic finding, the visual deficits are often uneven or unilateral because of asymmetric tumor growth.
Up to 30% of patients present with concomitant headache. Unlike other meningiomas in the region, however, early vision involvement means that patients frequently present early before other symptoms of mass effect develop; nausea, altered mental status, and seizure are uncommon. As a result, these tumors are usually relatively small (<3 cm) at the time of diagnosis. Their small size makes them suitable candidates for endonasal resection.
Evaluation
There are three important diagnostic modalities involved in the evaluation of suprasellar meningiomas: radiographic, ophthalmologic, and endocrinologic. Magnetic resonance imaging (MRI) (with thin slices through the optic canals) allows diagnosis of a suprasellar, extra-axial, vividly enhancing lesion with a dural tail. T2 sequences evaluate encasement of the distal internal carotid arteries and the proximal anterior cerebral arteries which are often displaced upward or laterally. These tumors should not be mistaken for a pituitary macroadenoma that requires a different resection strategy.
Careful evaluation of invasion into one or both optic canals, extent of lateral growth of the tumor, and the displaced position of the optic apparatus are critical to surgical planning and the need for aggressive intraoperative optic canal decompression. Increased T2/FLAIR signal in the basal frontal lobes may suggest an invasive or higher grade tumor. Computed tomography (CT) with thin cuts in multiple planes allows evaluation of the surrounding bony anatomy and air sinuses in order to assess the patient’s endonasal operative corridor.
Small paranasal sinuses and dominant anterior extension of the tumor may require impractical steep working angles to expose the anterior edge of the tumor. The CT scan also determines the extent of tumor-infiltrated hyperostosis. Calcified tumors are significantly more technically challenging to decompress and dissect endonasally.
Preoperative formal visual field and dilated fundoscopic examinations document a preoperative baseline and provide a reference point when monitoring for future recurrence, especially within the optic canal. More posteriorly situated tumors are prone to cause hypothalamic-pituitary dysfunction, which should be documented, and when necessary, corrected before surgery.
Other similar suprasellar lesions include a variety of pathologies that may be extrinsic (craniopharyngioma, germ cell tumor, metastasis, epidermoid cyst), intrinsic (hypothalamic or optic glioma, pituitary macroadenoma), or bony (giant cell tumor, aneurysmal bone cyst). Vascular studies (CT or MR angiography) can rule out atypical aneurysms and further define the surrounding displaced normal vasculature.
Indications for Surgery
Small, incidental lesions may be observed with serial imaging and eye exams. The role of radiosurgery is limited because of the close proximity of the mass to the optic apparatus. When ophthalmologic findings confirm mass effect on the optic nerves, chiasm, or tracts, surgery is recommended, both to prevent further visual deterioration and to potentially recover visual function.
Even in the presence of long-standing (>12 months) symptoms, improvement occurs in the majority of patients. Patients with mono-ocular blindness may occasionally recover some function.
Preoperative Considerations
With widespread adoption of endoscopic techniques, there is also growing controversy regarding the superiority of the endonasal over the transcranial route.
I have abandoned the transcranial route for more than 90% of tuberculum sellae meningioma surgeries in favor of the transnasal corridor. It is ideal to approach a subchiasmatic lesion through a subchiasmatic route. The transcranial route demands manipulation of the optic apparatus and its retraction to circumdissect the tumor.
Ultimately, the local pathoanatomy and the surgeon’s experience determine which tumors are suitable for the endonasal approach. Smaller lesions (<3-4 cm) without lateral extension beyond the optic nerves are ideal candidates.
Likewise, although the endonasal corridor may be narrow at certain coronal planes, it is possible to both see and remove tumor lateral to these limits based on the fish-eye field of view of the endoscope and the use of angled endoscopes and angled instruments.
Although the lateral limits of the corridor are formed first by the lamina papyracea, and then the carotid siphon in the cavernous sinus and the lateral opticocarotid recess around the sellae, as well as the carotid bifurcation in the suprasellar cistern, tumors that extend slightly beyond these limits can often be rolled toward the center once adequate internal debulking is performed.
I do not consider vascular encasement a contraindication to endoscopic surgery since endonasal microsurgical techniques allow atraumatic vascular dissection. Optic canal involvement is not a contraindication, and in fact, opening and exploration of the optic canal is required based on the preoperative imaging and intraoperative findings.
Figure 2: These images show tumors that are amenable to the endonasal (upper images) and transcranial (lower images) routes. Large tumors extending lateral to the optic nerves (lower images) are more reasonable candidates for a transcranial approach.
ENDOSCOPIC TRANSNASAL MICROSURGICAL RESECTION OF PARASELLAR MENINGIOMAS
The technical nuances for skull base parasellar craniotomy/osteotomy are discussed in the Endoscopic Expanded Transnasal Approach chapter.
Figure 3: Tuberculum sellae meningiomas attach to the dura along the posterior planum, tuberculum sellae, diaphragma sellae, and anterior sellar wall. Note the anterior tumor extension along the planum, invasion into the optic canals, and displacement of the optic chiasm superiorly and posteriorly (inset image). The amount of required bone work/craniectomy is demonstrated in blue.
Key anatomic considerations to consider include the size of the sphenoid sinus and tuberculum/suprasellar notch anatomy, the relative position of the optic chiasm and pituitary stalk, parasellar extension of the tumor or encasement of neurovascular structures, and degree of tumor calcification.
Patient selection is one of the most important factors for achieving good outcome, and an overzealous use of the transnasal corridor for poorly selected patients often leads to disappointing results.
Figure 4: Osteotomy extends slightly over the carotid arteries bilaterally and the optic canals. Wide bony exposure is mandatory to avoid blindly dissecting or pulling on the tumor. The anterior extent of bony removal is defined by navigation and the posterior extent reaches to the level of the mid sellar floor. To simplify skull base reconstruction at the end of the procedure, the entire floor of the sella should not be removed. The tumor is often easily dissected away from the pituitary gland/diaphragma sellae, obviating the need for a generous exposure posteriorly.
Bone removal extends along the planum to the anterior extent of the tumor and its dural tail. This osteotomy can be most challenging technically because of the steep and acute working angles; the drill is used generously at this juncture using 30 degree angled endoscopes. The dura and the intercavernous sinus are cauterized using bipolar electrocautery. This maneuver devascularizes the meningioma.
The dura is opened in a cruciate fashion and resected where involved with the tumor.
INTRADURAL PROCEDURE
Resection of fibrous, calcified or adherent tuberculum sellae meningiomas can be among the most demanding microsurgical transnasal procedures and an intimate familiarity with the endoscopic methods is mandatory before undertaking the resection of these tumors.
Graded advancement and the steep learning curve in endoscopic microsurgical techniques involve first proficiency in pituitary adenoma and craniopharyngioma surgery before endonasal removal of meningiomas is attempted.
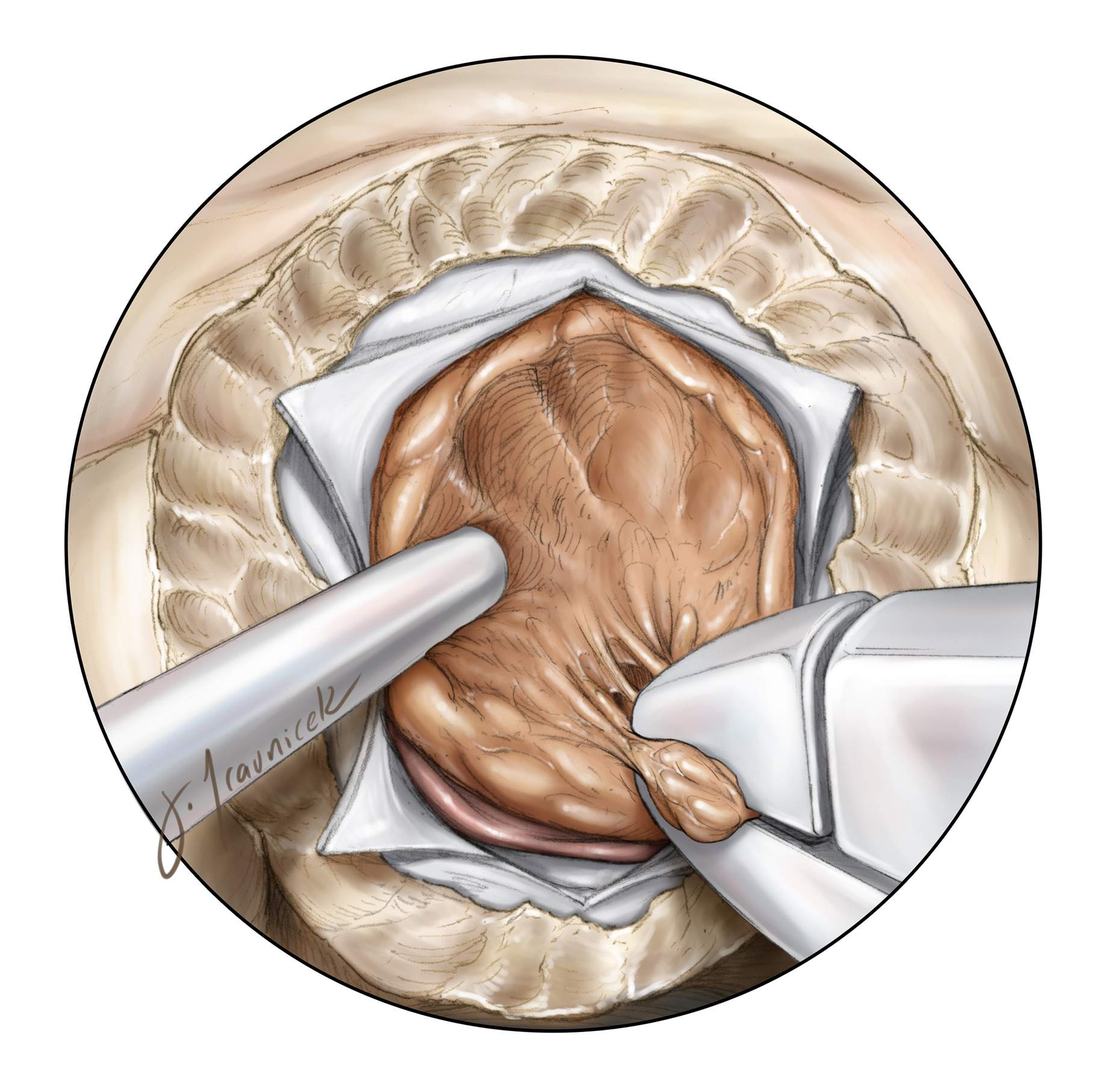
Figure 5: Upon wide dural opening, the inferior and posterior tumor margins are defined. Insufficient exposure and bone removal can lead to high-risk retraction and blind maneuvers with attended risks. Mobilization of the inferior pole allows me to appreciate the depth of the tumor and the need for further internal debulking without inadvertently violating the posterior capsule. Excessive traction on the capsule can be transmitted to the pituitary stalk or optic nerves and is not safe.
Figure 6: The first step after the exposure and tumor devascularization is internal decompression. Enucleation is performed using a number of instruments depending on the tumor’s consistency, including suction, pituitary rongeurs, and/or an ultrasonic aspirator.
An angled endoscope (30 degrees) allows improved visualization and resection of the anterior tumor bulk. A key advantage of the endonasal approach is that it allows the surgeon to expose and debulk the tumor early in dissection to achieve timely optic apparatus decompression before manipulating the nerves and chiasm. In contrast, the transcranial approach reaches the tense tumor after manipulation of the compressed optic apparatus.
Figure 7: Once internally debulked, the anterior tumor margin is identified. This step ensures that an adequate amount of planum has been removed. Furthermore, this is the safest part of the tumor capsule to dissect in order to understand the safe limits of tumor enucleation. The operator can then perform more aggressive debulking while following the anterior tumor margin before moving to the more delicate dissection maneuvers along the inferior and lateral margins.
Preoperative radiographic studies estimate the location of the A2 branches and perforators relative to the tumor; these vital vessels are encountered at this point along the anterior superior aspect of the capsule. Careful bimanual sharp dissection within the peritumoral arachnoid planes is essential. Blind pull on the tumor is not recommended.
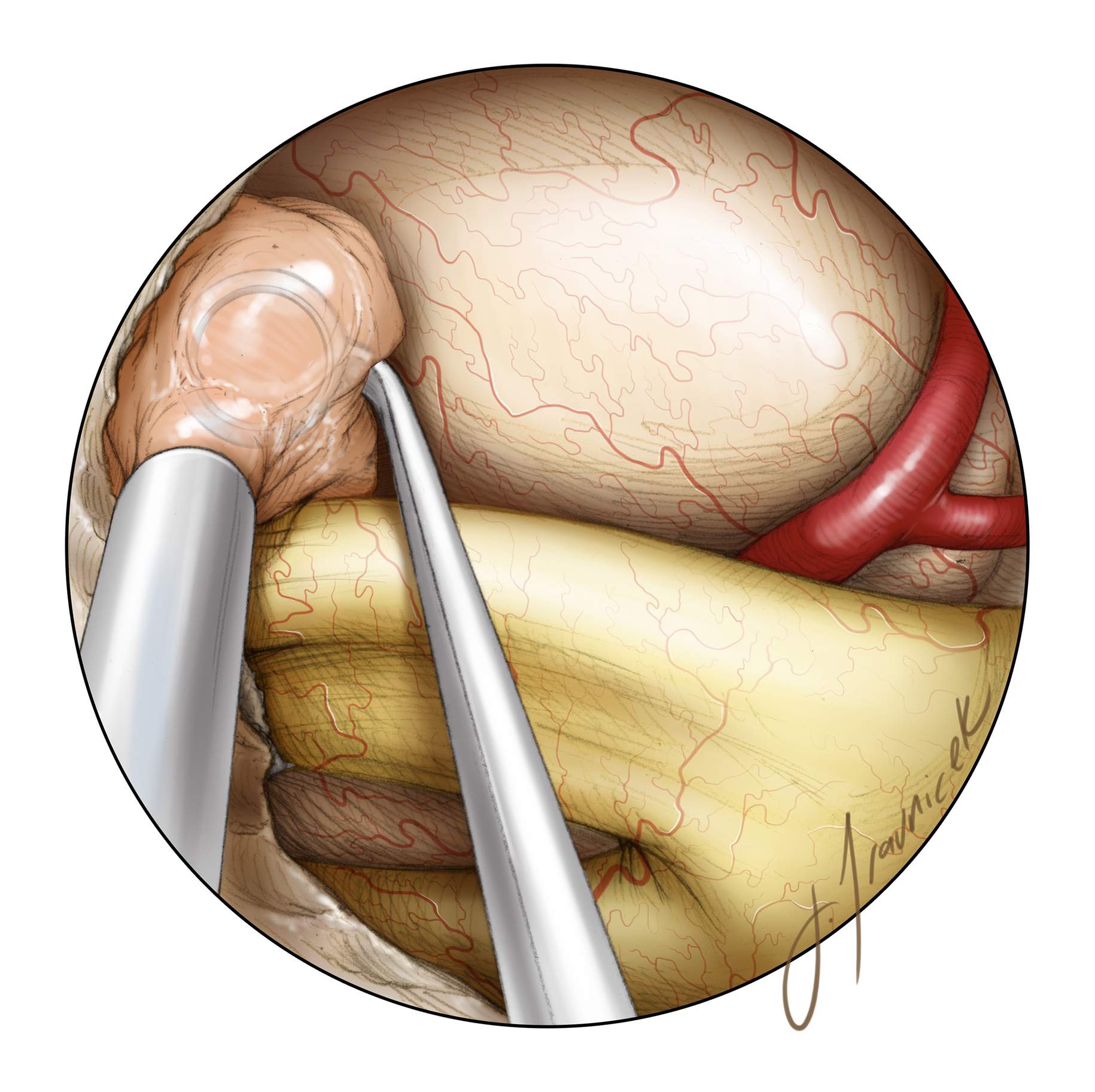
Figure 8: The inferior pole of the tumor often involves, or is based on, the dura of the diaphragm itself. The diaphragm is resected sharply where involved, avoiding injury to the pituitary stalk (inset image). Inferior tumor growth into the sellae often overlies a compressed pituitary gland that is usually posteriorly displaced. This margin is sharply dissected, often exposing the interpeduncular cisterns. The superior hypophyseal arteries will be displaced posteroinferiorly by the tumor and must not be injured.
Figure 9: One of the most common causes of postoperative visual deterioration is injury to the perforators of the optic chiasm, including the branches of superior hypophyseal artery. Careful preservation of en passage perforators will avoid disappointing postoperative visual complications. In this intraoperative photo, the tumor is elevated using the suction device (top of the image). The perforating vessels leading to the tumor versus to the optic apparatus should be clearly distinguished. The pituitary stalk, encased in the arachnoid layers, is evident under the chiasm.
Figure 10: Following the anterior and inferior tumor margins, further central tumor removal is completed. If adequate central decompression has been performed, the residual central tumor can be gently dissected away from the optic apparatus, with all arachnoid attachments sharply separated. This maneuver can be used to collapse the tumor medially, away from the optic nerves, mobilizing the lateral extents of the mass.
Figure 11: Angled instruments and endoscopes allow further mobilization of the lateral tumor capsule under direct vision. Centrally, the basilar and posterior cerebral arteries are visualized. The tumor should be gently moved into the central resection cavity rather than resected in situ attached to the optic nerves. A 30-degree scope is used to look behind the residual tumor to inspect any attachments to the delicate vasculature of the optic nerves, the posterior cerebral arteries, or the third cranial nerves, so as to avoid unnecessary traction on these structures.
Figure 12: Next, the optic canals should be inspected using an angled scope. If radiographic canal involvement was seen preoperatively, the medial wall of the optic canal has already been removed. If unexpected intraoperative invasion is found, the floor of the canal can be opened at this point with fine rongeurs and/or a high-speed drill.
Figure 13: Once the bony canal has been opened, the dura of the optic nerve is incised with a sickle knife. The dural opening is made anteriorly to avoid injury to the inferomedial ophthalmic artery. A microdoppler probe may help localize this vessel if its entry point cannot be clearly seen.
Figure 14: Angled scopes allow free inspection of the opened optic canal. Tumor removal should not be attempted until the dura of the optic nerve has been released; this maneuver prevents pinching the nerve against the dural edge during intracanalicular tumor mobilization. The arachnoid plane is then developed and the tumor rolled off the nerve before its removal.
Figure 15: This tuberculum sella meningioma (first row) underwent endonasal removal. The extent of bone removal is shown; note the generous osteotomy along the planum to make sure the anterior margin of the tumor is easily reachable (second row, left image). Only the anterior half of the floor of the sella is resected. Bony removal over the optic canals allowed early release of the nerves (second row, right image). The pituitary stalk was identified upon tumor debulking and its mobilization (third row, left image-purple arrow). Following tumor removal, angled endoscopes allowed exposure and thorough inspection of the right and left optic nerves through their foramina. The origin of the right ophthalmic artery is also apparent (third row, right image).
Closure
Removal of the bone over the optic canals presents special challenges in reconstruction of the skull base. Fear of a challenging reconstruction should not lead to inadequate bone removal before tumor resection.
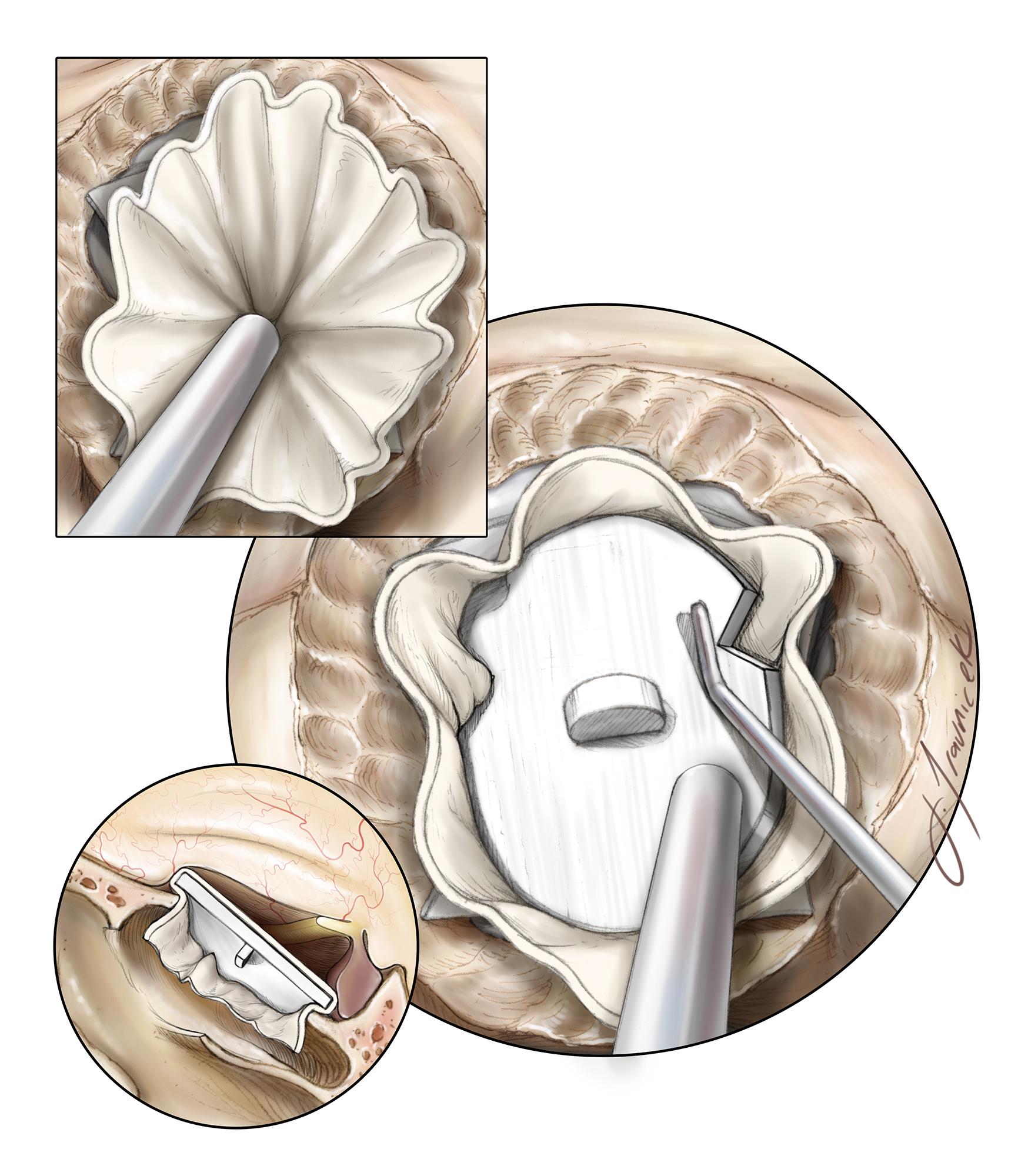
Figure 16: Once tumor resection is complete and the involved dura has been resected and/or cauterized, a gasket closure technique is performed by first placing an oversized dural substitute or fascial graft over the defect, followed by a countersunk rigid implant. Depending on the lateral extent of the bone opening, notches are cut into the implant to avoid contact with the optic nerves. The initially harvested nasoseptal pedicled mucosal flap is then placed over the seal and covered with fibrin glue.
Please see the Endoscopic Expanded Transnasal Approach chapter for further details about skull base reconstruction and closure.
Endoscopic Tuberculum Sella Meningioma: Steps for Tumor Resection
Postoperative Considerations
The lumbar drain is discontinued 2 to 3 days after surgery. A postoperative MRI is routinely obtained and has an important role in the learning curve of the surgeon to improve the extent of resection for future patients. Steroids may be slowly weaned as tolerated. Prophylactic anticonvulsants are administered perioperatively, but tapered off 1 week after surgery if the patient has not suffered from a seizure.
Monitoring of urine output and serum sodium allow management of temporary postoperative diabetes insipidus. Standard endonasal precautions are recommended to the patient, including avoiding of nose-blowing, straw use, and unnecessary bearing down.
Pearls and Pitfalls
- Early central tumor decompression avoids retraction on the optic apparatus during subsequent dissection of the tumor capsule.
- Angled endoscopes allow inspection of critical neurovascular structures once tumor margins are identified, especially along the lateral capsule that is in the operator’s blind spot.
- Inspection of the optic canals should always be conducted because canal invasion by the tumor may not always be visible on the preoperative MRI.
- Preservation of fine subchiasmatic perforators is critical for avoiding visual deterioration.
Contributor: Charles Kulwin, M.D.
References
Kulwin C, Schwartz TH, Cohen-Gadol AA. Endoscopic extended transphenoidal resection of tuberculum sellae meningiomas: nuances of neurosurgical technique. Neurosurgical Focus. 35:E6, 2013.
Please login to post a comment.