Paramedian Supracerebellar Craniotomy Free
This is a preview. Check to see if you have access to the full video. Check access
Paramedian Supracerebellar Craniotomy for Brainstem Pilocytic Astrocytoma
General Considerations
A variation of the supracerebellar craniotomy is the paramedian supracerebellar craniotomy, which has some features in common with the midline supracerebellar craniotomy. Because these procedures are two variations of the supracerebellar craniotomy, some information is repeated in these two chapters.
The supracerebellar craniotomy is one of the most underutilized approaches in neurosurgery. Its flexibility as a far-reaching corridor deserves much more exploration.
The midline bilateral suboccipital supracerebellar route is traditionally designed for exposing pineal region tumors. The limitations of this approach include limited lateral or inferior visualization caused by the angle of the tentorium and the obstructive apex of the culmen, respectively. Almost all midline bridging vermian veins are invariably sacrificed, and this maneuver is not without risks.
I recently abandoned the midline bilateral supracerebellar route in favor of the unilateral paramedian alternative even for accessing large midline pineal region tumors. I prefer the left-sided paramedian supracerebellar approach to avoid the dominant transverse sinus. In this approach, the trajectory over the lateral cerebellum or the quadrangular lobule is more direct and less steep versus the route over the apex of the culmen. The use of the paramedian corridor allows the surgeon to spare most of the midline vermian veins.
I believe the advantages of the paramedian versus a midline suboccipital craniotomy are similar to those of a frontolateral or pterional craniotomy versus a bifrontal craniotomy for resection of large olfactory groove meningiomas.
In this chapter, I focus on the paramedian supracerebellar approach that provides all of the necessary operative access to the pineal, posterolateral mesencephalon, posterior third ventricle, and the posterior basal temporal lobe. The traditional midline supracerebellar approach is described in its own dedicated chapter.
Figure 1: The paramedian supracerebellar approach has numerous advantages over the midline approach: 1) the craniotomy is less invasive and does not place the frequently more dominant right transverse sinus and torcula at risk, 2) only one cerebellar hemisphere is manipulated, 3) the vermian bridging veins are often protected, and 4) the lower slope of the lateral cerebellum provides a more inferior trajectory to the inferior pole of the tumor. Repeat surgery may be performed through the intact contralateral supracerebellar route.
Indications for the Approach
The supracerebellar approach is useful for exposing pineal region tumors such as germ cell tumors, pineoblastomas, astrocytomas, and other rare lesions such as meningiomas, epidermoid tumors, and pilocytic astrocytomas. Importantly, this is an alternative route and my preferred approach for resection of posterior third ventricular tumors because gentle manipulation of the pulvinar is well tolerated by patients. Vascular lesions such as tentorial arteriovenous fistulae and malformations are also amenable to this approach.
Other intraparenchymal posterolateral mesencephalic lesions such as cavernous malformations and pilocytic astrocytomas may be reached through this route. Distal superior cerebellar artery aneurysms may also be accessed.
Figure 2: Large lesions in the pineal region (left image) and posterolateral mesencephalon (right image) can be readily exposed through the paramedian supracerebellar route.
The transtentorial extension of the paramedian supracerebellar operative corridor is innovative. Wide sectioning of the tentorium through the supracerebellar space allows removal of the supratentorial extension of petrous apex meningiomas and avoids subjecting the patient to a second-stage subtemporal surgery. Posterior hippocampal cavernous malformations, arteriovenous malformations, astrocytomas, and metastasis may be resected through this approach.
This route is ideal for excising medial tentorial meningiomas while obviating the need for temporal lobe retraction to reach the medial tentorium through the subtemporal pathway. Therefore, the transtentorial modification may be used to resect a completely supratentorial tumor through an infratentorial craniotomy.
Figure 3: Transtentorial petrous apex meningiomas (upper images) and medial tentorial meningiomas (lower images) may be resected with a single-stage surgery using the paramedian supracerebellar transtentorial route.
Preoperative Considerations
Preoperative magnetic resonance images will disclose the extent of the tumor and the need for a combined or transtentorial corridor. Obstructive hydrocephalus requires preparation of the Keen’s point or a preoperative frontal ventriculostomy. The paramedian linear incision can readily uncover the bony area corresponding to the burr hole for the Keen’s point. Germ cell tumors should be excluded using serum and cerebrospinal fluid markers.
I use the modified park-bench position for patients during this procedure. Cerebrospinal fluid drainage through the lumbar drain (in the absence of obstructive hydrocephalus) provides further decompression for mobilization of the cerebellum.
The transverse and sigmoid sinuses may have slightly variable courses and their preoperative study can enhance the safety of the craniotomy. Factors such as a steep tentorial angle and a very obese patient with a short neck, although not contraindications to the use of the supracerebellar route, can make the operation more challenging. In these situations, the patient’s neck flexion may ameliorate the difficult working angles over the cerebellum, and it is recommended that the patient be placed in the sitting position.
Magnetic resonance images can provide critical information about the relationship of the deep venous structures (vein of Galen, basal vein of Rosenthal, internal cerebral veins, and straight sinus) to the operative trajectory and tumor. Occasionally, posterior thalamic and vermian tumors mimic primary pineal region masses and displace the diencephalic veins posteriorly; this configuration is a potential contraindication for use of the supracerebellar approach. The degree of tumor infiltration through the surrounding critical neural structures (e.g., midbrain, thalamus) must be studied before surgery.
Operative Anatomy
Click here to view the interactive module and related content for this image.
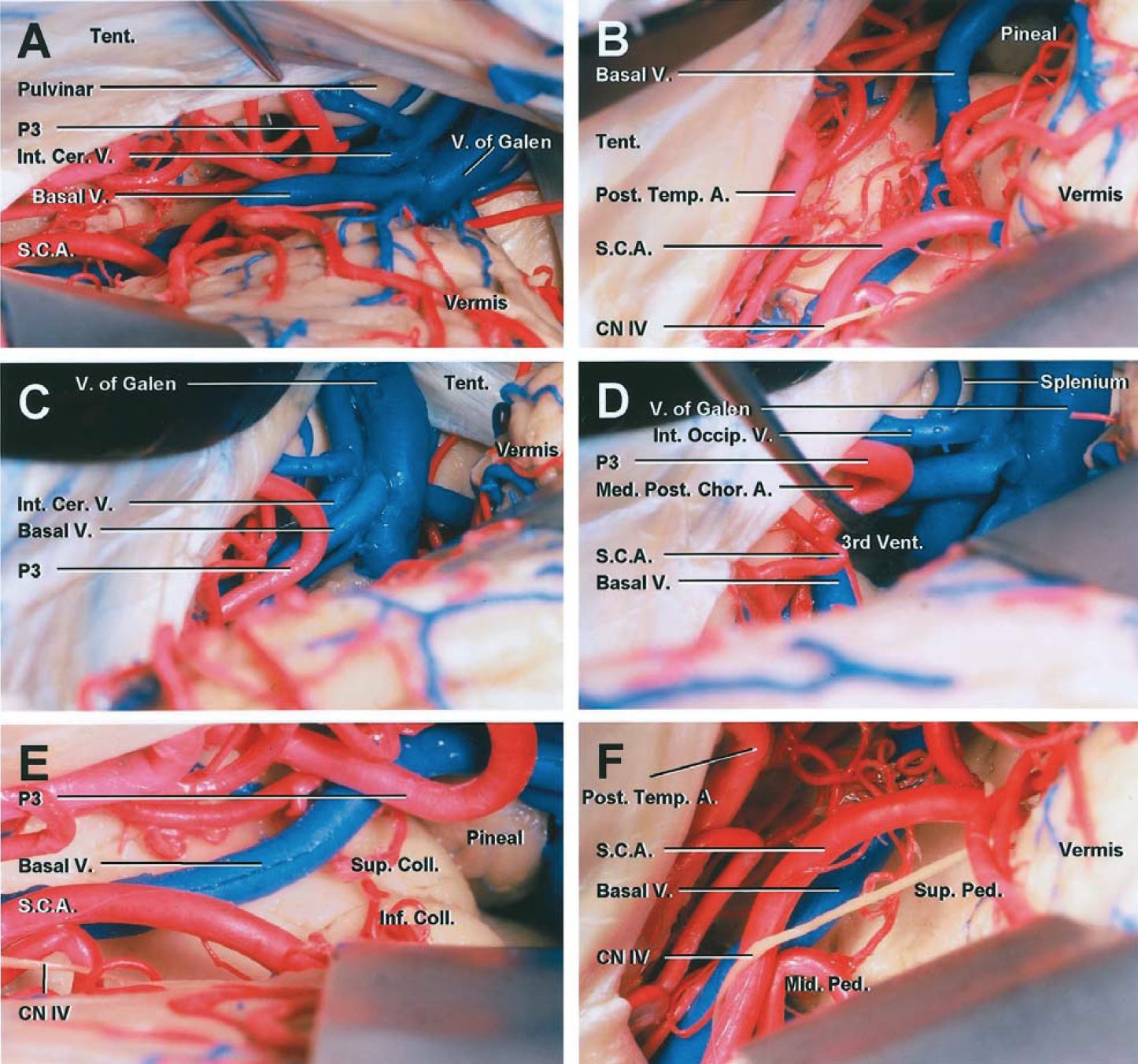
Figure 4: The regional anatomy for approaching the pineal region is shown. Note the relationship of the posterior basal temporal lobe and occipital lobe to the pineal area (A). These supratentorial regions become available after transection of the tentorium through the supracerebellar route. The surface anatomy of the cerebellum (B) and posterior diencephalon (C and D) are shown. The vascular arterial (E) and venous (F) anatomy are evident (E)(Images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 5: Higher magnified images of the cerebrovascular anatomy within the pineal region through the paramedian supracerebellar approach are shown. The splenium is anterior to the vein of Galen. As the operator follows the contours of the tentorium toward the pineal region, he or she may be led to inadvertently dissect around the vein of Galen. This error must be avoided and the operative trajectory readjusted inferiorly toward the pineal region. Note the generous view of the pineal region through the lateral trajectory (E)(Images courtesy of Rhoton, AL).
Figure 6: Compared with the midline approach (left image), the paramedian supracerebellar approach (right image) avoids most of the midline vermian bridging veins.
Figure 7: The midline route (left image) depends upon retraction of the culmen, whereas the paramedian route (right image) reaches over the more inferiorly situated lateral cerebellum. Cranial nerve IV is situated at the lower edge of the dissection field.
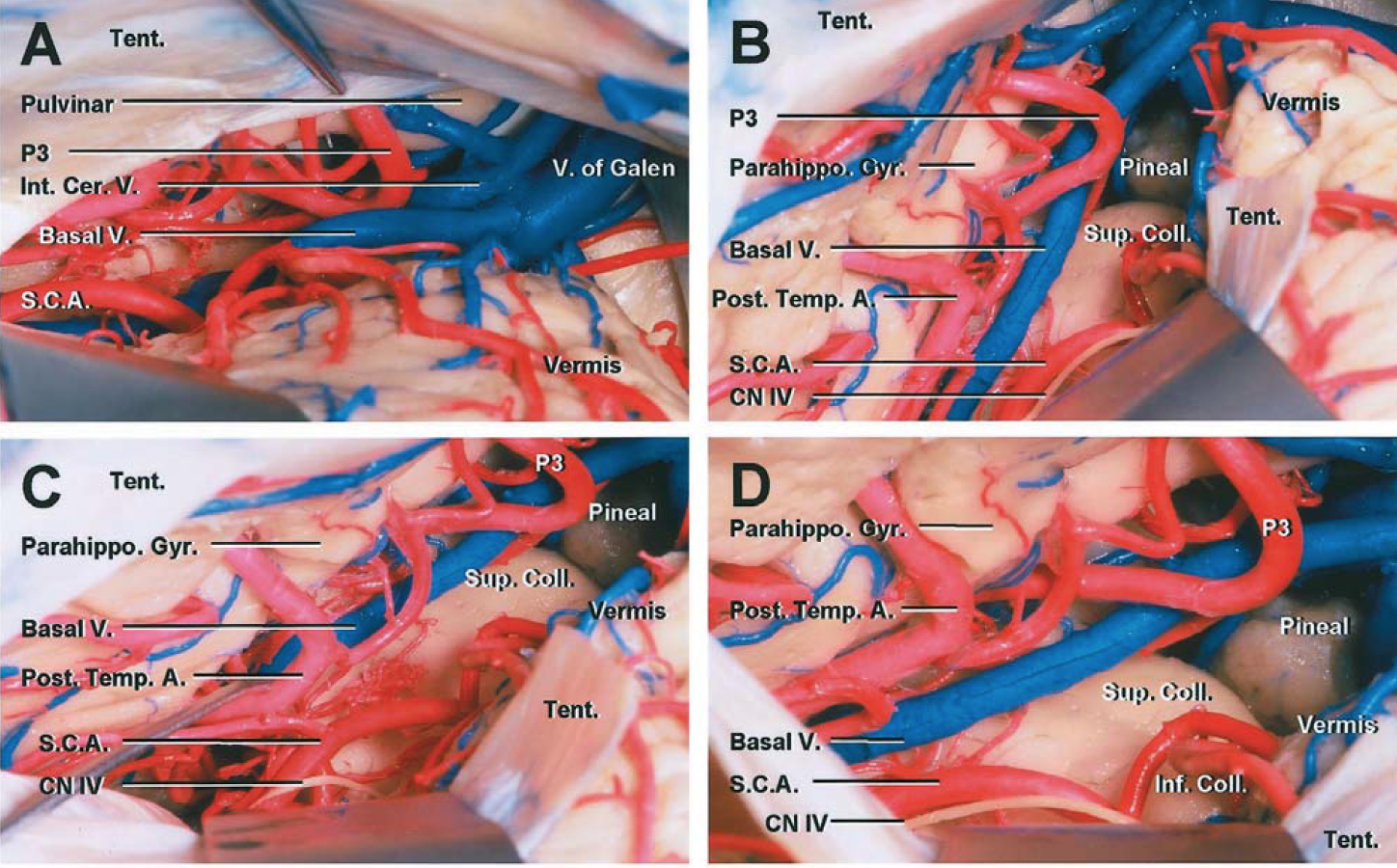
Click here to view the interactive module and related content for this image.
Figure 8: Sectioning a window of the left tentorium through a paramedian supracerebellar craniotomy exposes the posterior ambien cisterns, basal temporal lobe, and the relevant arterial anatomy (B, C, and D). Note the generous exposure of the posterior parahippocampus and distal posterior cerebral artery branches through this route (Images courtesy of AL Rhoton, Jr).
PARAMEDIAN SUPRACEREBELLAR CRANIOTOMY
As stated previously, I prefer to use the paramedian supracerebellar approach to the pineal, posterolateral mesencephalon, posterior third ventricular and posterior basal temporal territories with the patient placed in a modified park-bench position.
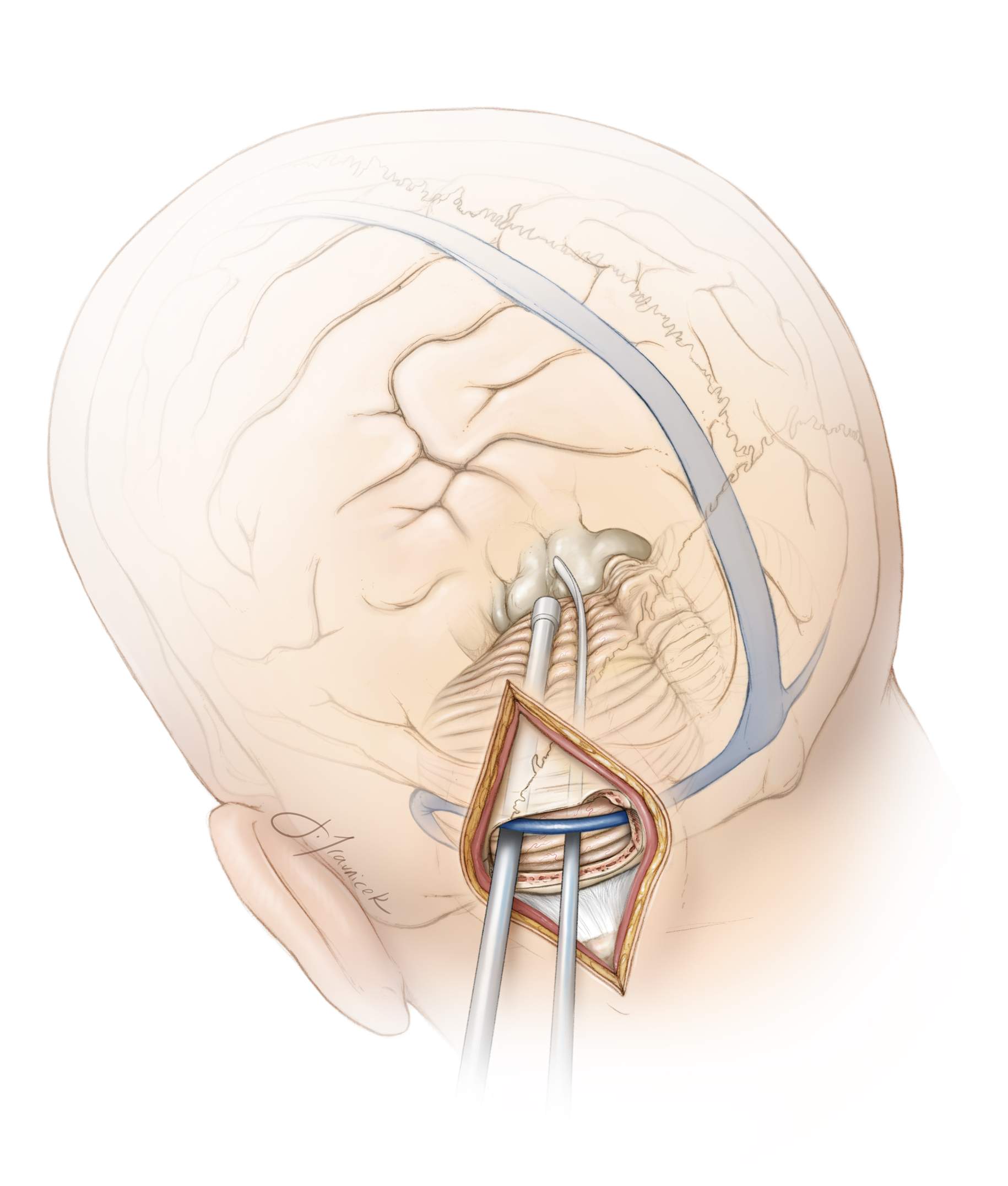
Figure 9: The left-sided suboccipital craniotomy protects the torcula and the often more dominant right-sided veins and dural sinuses, including the transverse sinus. A skull clamp is used with the patient’s neck flexed and head turned slightly (15-20 degrees) toward the floor. The location of the tumor is marked with X on the upper image.
The patient’s ipsilateral shoulder is allowed to fall forward and is taped away from the surgeon’s working zone. Intraoperative neuronavigation may be used to identify the location of the midline as well as the transverse and sigmoid sinuses. A paramedian vertical linear incision is made halfway between the inion and the mastoid groove. This incision extends one-third above and two-thirds below the transverse sinus and is about 7–8 cm in length. Note the Keen’s point (arrow, lower left image) is within the upper edge of the incision.
Figure 10: A single burr hole is made at the inferior edge of the transverse sinus, approximately 2 cm lateral to the midline and torcula. A small bone flap is elevated while the entire width of the transverse sinus is exposed to allow room for later rostral mobilization of this sinus. The dura is opened as a single curved flap based on the sinus. Two retraction sutures are placed along the posterior aspect of the tentorium to mobilize and gently rotate the transverse sinus superiorly to expand the operative space through the supracerebellar corridor.
Gradual release of cerebrospinal fluid through the lumbar drain or ventriculostomy catheter allows gentle caudal mobilization of the lateral cerebellar hemisphere.
Figure 11: Traditional bone removal and dural opening restrict the working angles of the surgeon and limit the corridor within the supracerebellar space (top image). Placement of a fixed retractor on the tentorium, despite skeletonization of the sinuses, does not significantly improve the operative corridor. In fact, it may interfere with the working angles of the instruments (middle image). The retraction sutures anchored on the tentorium elevate the tentorium, mobilize/rotate the transverse sinus, and expand the operative corridor (bottom image).
Figure 12: One or two paramedian bridging veins may have to be sacrificed. Large midline bridging veins are left intact. Note the retraction sutures placed through the posterior tentorium. These sutures gently rotate and mobilize the transverse sinuses superiorly. Microdoppler ultrasonography can confirm patency of the sinus and gauge the safe degree of retraction on the sinus.
Figure 13: Dissection of the arachnoid membranes over the medial dorsolateral mesencephalon will mobilize the cerebellum downward and open the corridor toward the midline. Cranial nerve IV is evident in the inferolateral corner of the exposure.
Figure 14: Upon opening the arachnoid membranes over the tumor, the surgeon can begin microsurgical removal of the tumor. This “cross court” trajectory is beneficial since the tentorial surface of the cerebellum is highest along its anteromedial apex and slopes down laterally. Therefore, the lateral paramedian operative corridor provides a more inferior trajectory to the posterior midline and posterolateral mesencephalon as compared with the midline trajectory to access the inferior extension of large pineal region tumors.
Dynamic retraction of the cerebellum using the suction apparatus allows exposure and resection of the inferior extent of the tumor without the use of fixed retractors. The suction apparatus allows a more controlled expanded view of the working zone at the exact location of the dissection, whereas the wide blade of the retractor may at times compromise the deep exposure because of its less flexible vector of retraction. Generous exposure of the contralateral tectum is readily available through this unilateral working channel and near the falx cerebelli.
SUPRACEREBELLAR TRANSTENTORIAL APPROACH
This modification of the approach can also be performed with the patient in the park-bench position.
Figure 15: Note the role of this route for resection of medial tentorial tumors. Cranial nerve IV must be protected along the lateral edge of the incisura during tentorial transection (inset image). Incision along the red hashed line will sacrifice the nerve—incision along the black hashed line is appropriate. Alternatively, a “T” shaped incision may be made within the tentorium for intraparenchymal lesions within the posterior basal temporal lobe.
Figure 16: Early exposure of the posterior brainstem and surrounding neurovascular structures at the tentorial incisura allows for their protection by microdissection away from the tumor before significant tumor debulking is performed and the surgical field is obscured by bleeding. Extra-axial tumors can be devascularized early in surgery through cauterization of the undersurface of the tentorium.
Figure 17: A generous portion of the tentorium is then incised from the petrous ridge to the midline while identifying and preserving the trochlear nerve along the entire anterior edge of the tentorium. Occasional bridging veins draining the occipital lobe and entering the superior aspect of the tentorium may be sacrificed. The medial tentorial cut should preserve the straight sinus and its tributaries. Venous lakes may be present and venous bleeding through the leaflets of the tentorium should be controlled using thrombin soaked gelfoam packing. Bipolar cauterization will exacerbate the bleeding by shrinking and tearing the tentorial edges.
Sectioning of the tentorium as described above will further devascularize the tumor and allow a relatively bloodless field to debulk the tumor and microsurgically mobilize it from the surrounding cortex.
Figure 18: This tentorial resection creates a wide corridor to the basal occipital and posterior/medial temporal regions. An intra-axial tumor in this region can be similarly resected.
Medial Tentorial Meningioma: Supracerebellar Transtentorial Approach
Closure
The dura must be closed in a watertight fashion because the occurrence of postoperative cerebrospinal fluid fistulae is a significant risk after tumor operations within the posterior fossa. I prefer to avoid using an allograft to reconstruct the dural defect and instead use a piece of pericranial autograft.
The bone may be replaced using cranial plates. I avoid placing the deep neck muscles under significant tension and minimize their strangulation by deep sutures to protect the patient from muscle necrosis and uncontrolled postoperative pain. The neck muscles are gently approximated. The fascia is closed in a watertight fashion.
Postoperative Considerations
The patient is observed in the intensive care unit for a day or two after surgery and then transferred to the ward. Steroids are administered prophylactically to minimize the risk of aseptic meningitis. If preoperative hydrocephalus was present and a ventricular catheter was implanted intraoperatively, this catheter should be left in place during surgery and weaned off after surgery.
Aggressive retraction of the cerebellum can lead to retraction edema. This can be seen on postoperative imaging and can ultimately cause symptomatic posterior fossa tension and a need for decompression. Therefore, caution should be exercised during dural closure and bone flap replacement. If the brain appears swollen, the dural closure should not cause more tension and the bone flap should not be replaced. This brain swelling can be potentially compounded by partial transverse sinus thrombosis and paravermian vein sacrifice.
Pearls and Pitfalls
- Compared with the bilateral midline suboccipital supracerebellar approach, the paramedian supracerebellar approach is less invasive and provides adequate exposure for resection of large pineal region tumors.The paramedian alternative places bilateral dural venous structures and cerebellar hemispheres at less risk.
- The use of tentorial retraction sutures to rotate and elevate the transverse sinuses expands the supracerebellar operative corridor.
References
Kulwin C, Matsushima K, Malekpour M, Cohen-Gadol AA. Lateral supracerebellar infratentorial approach for microsurgical resection of large midline pineal region tumors: techniques to expand the operative corridor. J Neurosurg. 2015.
Please login to post a comment.