Low Flow Revascularization
This is a preview. Check to see if you have access to the full video. Check access
Middle Cerebral Artery (M2) Dissection: STA-MCA Revascularization
Please note the relevant information for patients undergoing cerebral bypass surgery is presented in another chapter. Please click here for patient-related content.
Woringer and Kunlin performed the first documented extracranial-intracranial (EC-IC) bypass in humans in 1962. This procedure was performed between the common carotid and the intracranial arteries. Unfortunately, the patient died from pneumonia during the postoperative period. However, this experience paved the way for the new era of cerebral revascularization.
Yasargil performed the first successful superficial temporal artery to middle cerebral artery (STA-MCA) bypass in humans in 1967. Since then, this procedure has evolved in its technique and indications. The current main indications for the STA-MCA bypass include:
- Cerebral ischemia
- Moyamoya disease
- Complex intracranial aneurysms
- Complex skull base tumors
Because there is much controversy about the true indications of this form of bypass, I will briefly review the history and implications of recent trials on the application of this operation.
Cerebral Ischemia
The STA-MCA bypass has played an important role in the management of cerebrovascular occlusive diseases such as internal carotid artery (ICA) stenosis or occlusion that are not amenable to carotid endarterectomy. Symptomatic ICA and MCA stenosis or occlusion became the earliest established indications for this treatment.
In 1977, Barnett and colleagues took on a large randomized controlled trial to study the value of the STA-MCA bypass (EC-IC bypass Trial) for managing cerebrovascular occlusive disease. Their results were published in 1985, indicating a failure of the bypass to provide benefit.
Despite these results, the trial demonstrated a greater than 95% graft patency rate, indicating the technical success of the operation. Notwithstanding the proper methodology of this study, many arguments ensued about the applicability of the results. Some of the most convincing arguments questioning this study were the investigator’s failure to identify the cause of ischemic events (embolic or occlusive), the lack of objective quantification of stenosis or cerebral perfusion and hemodynamics, the inclusion of predominately low-risk patients, and conduction of a large number of procedures outside the trial.
It became obvious that more rigorous preoperative selection criteria were necessary. After the trial, in spite of the questions about it, there was a substantial decrease in the rate of the STA-MCA bypass procedures performed for cerebrovascular occlusive disease.
The proponents of the above EC-IC Bypass Trial later emphasized the importance of hemodynamic factors in patient selection. Patients with hemodynamic failure Grade II, or “misery perfusion” may benefit from the STA-MCA bypass. Hemodynamic failure Grade II describes patients who have exhausted all compensatory mechanisms available to overcome the decrease in cerebral perfusion pressure from vascular occlusion.
This situation is characterized by increased regional mean transit time (rMTT), decreased regional cerebral blood flow (rCBF), and increased regional oxygen extraction fraction (rOEF) on positron emission tomography (PET). Such patients showed dramatic improvement in their rOEF and return to baseline values in the St. Louis Carotid Occlusion Study.
In 2006, a Japanese study group conducted a new trial, the Japanese EC-IC Bypass Trial (JET Trial), based on the above criteria as well as advanced imaging modalities. The JET Trial demonstrated a significantly improved 2-year outcome for surgical patients.
Despite these results, a North American trial, the Carotid Occlusion Surgery Study (COSS), completed in 2008, proved negative results for STA-MCA bypass surgery with a high 30-day morbidity among surgical patients and a nonstatistically significant decrease in 2-year morbidity.
These opposing results raised suspicions that the technical abilities of the surgeons involved and the length of time required for blossoming of the single-branch STA-MCA bypass could be factors in the discrepancy of the results. In light of these inconsistencies, a number of study groups, through case series or small trials, have proposed that double-barrel bypass, using both branches of the STA, may allow the benefit of the augmented flow of high-flow bypass without the high risk of reperfusion injuries.
In addition, advancement of endovascular techniques has led to another decline in the application and popularity of bypass surgery for ischemic disease. Nonetheless, STA-MCA has played a role in emergent revascularization for rare acute stroke patients with limited watershed infarcts who are not candidates for or who have failed endovascular therapy.
I continue to offer this form of revascularization to patients with carotid occlusion who suffer from progressive ischemic symptoms despite aggressive medical/antiplatelet therapy with documented evidence of misery perfusion on hemodynamic imaging.
STA-MCA Bypass for Carotid Occlusion
Moyamoya Disease
The second indication for the STA-MCA bypass is Moyamoya disease. Although its benefit in this disease has not been examined in randomized clinical trials, level III evidence has been sufficient to convince most clinicians of its benefit.
The reason behind this philosophy is the marked morbidity and mortality rate seen along the natural course of this disease and the almost immediate benefit following revascularization surgery. The natural history of this disease is associated with a significant debilitating morbidity or mortality within 2 years of the initial presentation in approximately 75% of children. Similar results occur in 20% of adults within a similar time frame.
Despite the establishment of a number of classification systems for staging the imaging findings of Moyamoya disease (the most widely used being the Suzuki classification), there is no proven correlation between the classification and surgical indications or prognosis.
The controversy with regard to symptomatic Moyamoya disease may not necessarily concern the concept of performing a revascularization procedure, but rather which procedure should be performed: direct or indirect (such as encephaloduroarteriosynangiosis, EDAS). Consensus for the most appropriate treatment for asymptomatic patients is lifestyle changes and medical management.
An ongoing trial, the Asymptomatic Moyamoya Registry (AMORE Trial) will provide more detailed knowledge regarding the long-term prognosis of asymptomatic patients with Moyamoya disease. The results could possibly lead to a change in the management choices for these patients.
It is important to note that there are a number of differences in the demographics of patients with Moyamoya disease among different ethnicities. In patients of Asian ethnicity, Moyamoya disease is mainly a primary disease with bimodal distribution in both children and adults, and hemorrhage is the more common presentation in adults. However, in Caucasians, Moyamoya disease more commonly occurs secondary to other diseases and processes, such as atherosclerosis and radiation exposure, and it tends to occur primarily in adults, presenting mainly with ischemia.
Many clinicians believe that the most appropriate treatment for children is indirect revascularization because of their fairly small-caliber vessels that make vascular anastomosis technically difficult, and also because of the high proliferative capacity in children, permitting efficient neovascularization.
However, in adults, the STA-MCA bypass is the more suitable choice because adults have large enough vessels for anastomosis, and some studies have shown better results with direct rather than indirect revascularization. The Japanese Adult Moyamoya Trial published in 2014 supported this hypothesis.
Furthermore, because the more common presentation in adults with primary disease is hemorrhage, an indirect technique would potentially be problematic as it increases neovascularization, adding to the risk of hemorrhage. There also have been some reports advocating the use of combined direct and indirect modalities for the treatment of such patients.
Revascularization is indicated in almost all patients with Moyamoya disease who present with TIA or stroke. Patients who have suffered from major strokes involving almost the entire MCA territory are not candidates as there is little remaining brain to protect with a bypass.
I recommend revascularization for Moyamoya-associated intracranial hemorrhage as long as there is evidence of hypoperfusion and reduced cerebrovascular reserve on perfusion imaging. The protective effect of bypass on the future risk of intracranial hemorrhage is debated.
The symptomatic side is revascularized first. The second surgery may be conducted as soon as a week later.
STA-MCA Bypass for Moya-Moya Disease
Complex Intracranial Aneurysms
There are two broad circumstances that demand the use of bypass for repair of cerebral aneurysms: 1) a temporary bypass for the procedures requiring prolonged interruption of blood flow, and 2) a permanent bypass for clip ligation of the aneurysms that require compromising the parent vessel or a major branch.
The common indications for permanent bypass in complex aneurysm surgery are:
- Broad-neck, large or giant aneurysms
- Absent neck (fusiform aneurysms)
- Atherosclerosis or calcification at the neck
- Thrombotic large or giant aneurysms
- Blister-like (dorsal paraclinoid aneurysms)
- Complex dissecting aneurysms
- Intracavernous giant aneurysms
- Traumatic/infectious pseudoaneurysms
However, in these and other situations, bypass is not the only option available for revascularization. Other possibilities include the use of multiple fenestrated clips for lumen reconstruction, endovascular stent-assisted coiling of the aneurysm, or proximal endovascular occlusion in patients with good collateral flow who undergo a successful balloon occlusion test (BOT). Note, however, that the results of the BOT can be quite unreliable.
Advanced endovascular methods including flow diversion technology have significantly narrowed the applications of revascularization for aneurysms.
Giant MCA (M2) Aneurysm: STA-MCA Bypass and Proximal M2 Occlusion
Complex Skull Base Tumors
Aggressive skull base tumors may invade the major vessels of the anterior circulation, and thus require sacrifice of certain indispensable vessels, demanding a bypass to maintain blood supply to the associated vascular territory.
Although some benign tumors, including meningiomas, can be gross totally removed, leaving the vessel intact, other more aggressive tumors cannot be dissected from the vessel with as much success. Although some surgeons may choose to proceed with subtotal resection of the tumor combined with treating the remaining portion with radiotherapy, others advocate sacrificing the vessel during aggressive tumor removal.
Proximal arterial occlusion without bypass is a possible alternative, provided a BOT is performed. This practice remains quite controversial, however, given that many patients have succumbed to stroke postoperatively despite a successful preoperative BOT. I recommend revascularization despite an encouraging BOT.
I advocate for subtotal resection followed by radiotherapy in most circumstances, because complete tumor resection is most often not possible due to the concurrent involvement of other vital structures such as the cranial nerves.
Preoperative Considerations
A number of imaging modalities are required to properly determine the need for a revascularization procedure, as well as for proper surgical planning.
The imaging sources are comprised of three main types: 1) structural imaging for assessment of the brain parenchyma, any mass effect and/or structural abnormalities; 2) angiography for visualization of the extracranial and intracranial components of the anterior circulation; and 3) perfusion or metabolic imaging, for evaluation of perfusion disturbances related to the underlying pathology or proximal vessel occlusion or sacrifice during surgery.
Various imaging modalities have been described for each of the above types. This chapter includes a discussion only of those commonly used in practice and relevant to the objectives of this chapter.
Structural Imaging
The most sensitive structural imaging modality currently used in clinical practice is magnetic resonance (MR) imaging. Proper use of the different imaging sequences is beneficial.
Diffusion weighted images (DWI) have a special value in cerebral ischemia for correlating the anatomic distribution of ischemic events with vascular territories. The presence of multiple ischemic foci in a single vascular territory raises suspicion of an occlusive or stenotic etiology, whereas random distribution of ischemic foci along different vascular territories holds a higher suspicion for embolic events. Infarcts at the border zones between the anterior cerebral and middle cerebral arteries as well as middle cerebral and posterior cerebral arteries are characteristic of hypoperfusion phenomenon.
In emergency situations, computed tomography (CT) imaging is very valuable for ruling out intracranial hemorrhage, as in patients with transient ischemic attacks and stroke or neoplastic apoplexy.
Angiographic Imaging
Angiography is required for diagnostic purposes, preoperative planning, and postoperative evaluation of graft patency. More specifically, angiography evaluates 1) the patency of the intracranial circulation and recipient vessels; 2) the patency of the external carotid circulation donor vessels; 3) the development of collateral circulation, which if sufficient may eliminate the need for revascularization; and 4) the diameter of the vessels that would be the expected sites of anastomosis, namely the frontal and parietal branches of the STA and the cortical branches of the MCA.
Although catheter-based angiography is the gold standard modality for vascular imaging, it is still somewhat invasive. Noninvasive modalities such as CT and magnetic resonance (MR) angiography have limited spatial resolution and are complicated by the bony artifact near the skull base. They also do not provide reliable information about the flow within the target vessels.
Perfusion and Metabolic Imaging
In cerebrovascular occlusive disease, perfusion imaging determines the success of collateral vasculature in overcoming the ischemic insults of the underlying vascular occlusion or stenosis. These imaging modalities include PET, xenon computed tomography, CT perfusion, MR perfusion and single photon emission CT.
As previously mentioned in the above discussion of the STA-MCA bypass trials, the sole use of angiographic findings in patient selection for revascularization surgery has been criticized. It is currently standard practice to use perfusion imaging to determine the status of reserve mechanisms for overcoming cerebral ischemia as a result of the decrease in cerebral perfusion pressure.
In order to indirectly assess such reserve mechanisms, four parameters are especially valuable: 1) regional cerebral blood flow (rCBF), 2) regional mean transit time (rMTT), 3) regional cerebral blood volume (rCBV), and 4) regional oxygen extraction fraction (rOEF).
A scheme of events has been involved in compensatory mechanisms associated with a decrease in cerebral perfusion pressure. This scheme divides the process into two stages of hemodynamic impairment. Stage I, also known as the autoregulatory stage, maintains normal rCBF values by arteriolar vasodilatation, resulting in an increase in regional mean transit time (rMTT) and regional cerebral blood volume (rCBV).
The second stage, or the hemodynamic failure stage, occurs at the point when autoregulatory processes have been mostly exhausted and sustaining oxygen requirements of the parenchyma are achieved by means of an increase in rOEF. An increase in rOEF is a significant independent risk factor for stroke. Although rOEF may also increase in a state of normal rCBV, the risk of stroke is still considered increased.
Not only is standard perfusion imaging valuable in the preoperative evaluation, but challenge tests may also be employed. The first challenge test is the acetazolamide challenge test. A vasodilatation stimulus, acetazolamide, would cause a steal phenomenon and ischemia among patients with exhausted autoregulatory vasodilatation; this is in contrast to improved rCBF in patients who have not reached maximal compensatory regional vasodilatation.
The results of the acetazolamide challenge test have been classified into three types: Type I patients have normal baseline cerebral blood flow (CBF) that increases after the acetazolamide challenge. Type II patients have areas of decreased CBF on baseline studies that improve after acetazolamide administration. Type III patients have decreased CBF at baseline and a paradoxic continued reduction in regional CBF after acetazolamide administration. Type III patients are thought to benefit the most from microsurgical revascularization.
The second challenge test is the balloon occlusion test (BOT). This test determines whether established collateral circulation can maintain adequate blood flow in case of proximal vessel sacrifice, as in aneurysm entrapment or tumor resection. However, despite a successful BOT, a significant proportion of patients develop ischemia postoperatively. Thus, revascularization as a standard practice after proximal vessel sacrifice, regardless of BOT results, is preferred.
Operative Anatomy
Understanding the microsurgical anatomy of the STA is a key factor in safe dissection of its branches for bypass procedures.
Figure 1: The STA is the smaller of the two terminal branches of the external carotid artery. It originates within the parotid gland and extends superiorly behind the neck of the mandible to pass superficial to the posterior root of the zygomatic process. Above this process, it divides into its two final branches, the frontal and the parietal. Near the zygomatic process, the temporal branches of the facial nerve and a number of concomitant veins cross over the STA (images courtesy of AL Rhoton, Jr).
The STA is also related to the auriculotemporal nerve, which runs behind the STA along its course. The main trunk of the STA has an inner diameter of about 1.8 ± 0.5 mm, whereas its terminal branches have an inner diameter of about 1.4 ± 0.5 mm. The minimum diameter of the donor vessel for direct bypass is 0.9mm.
The parietal branch of the STA is commonly the larger of the two terminal branches, and curves cranially and posteriorly to anastomose with the posterior auricular and occipital arteries, as well as the parietal branch of the STA on the opposite side. The parietal branch of the STA runs superficial to the temporalis fascia. The frontal branch runs cranially and anteriorly to anastomose with the supraorbital and frontal arteries.
STA-MCA REVASCULARIZATION
Familiarity with microsurgical techniques is pertinent in execution of this procedure.
Anesthesia, Monitoring and the Initial Steps
Patients with cerebrovascular ischemia have exhausted most of their cerebral reserve. Any marked change in blood pressure or ventilation can lead to devastating ischemic effects.
Accordingly, blood pressure should be tightly controlled with invasive monitoring and normocapnia should be maintained throughout surgery. A central venous catheter is placed. Electroencephalography (EEG), motor evoked or somatosensory evoked potential monitoring are recommended during temporary MCA branch clipping because of the vulnerability of these patients to the resultant ischemic insults. Any change in the potentials requires an immediate response with improvement in blood pressure parameters.
Further, during the period of temporary clipping and anastomosis, the patient might be placed under metabolic burst suppression using barbiturates or Diprivan (propofol). The patient’s blood pressure should be increased approximately 20% above baseline during that time. To avoid thromboembolic complications at the sites of anastomosis and temporary clipping, 2000 to 3000 units of heparin are administered intravenously 3 minutes before vascular occlusion.
The patient is situated in the supine position, with the head immobilized in a skull clamp. The head is turned 30° to 45° toward the contralateral side. I tilt the vertex of the head slightly toward the floor to use gravity to clear away the operative field of fluids during the performance of anastomosis.
After proper fixation, a miniDoppler probe is used for localization and marking of the route of the STA trunk and its frontal and parietal branches. The site is then properly prepared. Local injections of xylocaine/adrenaline are avoided to prevent spasm of the STA and its branches.
I strongly recommend the sitting position for the surgeon and properly positioned armrests to avoid arm fatigue and hand tremor. These comfort measures advance the pleasure of performing microsurgery for me.
Figure 2: The patient’s head is fixed in a skull clamp and rotated approximately 45 degrees toward the contralateral side; the vertex is tilted slightly toward the floor. Using a Doppler probe, the route of the STA is mapped and the incision over this route is marked. Care must be taken to differentiate the pulsating arterial flow from the surrounding continuous venous hum.
Figure 3: The incision typically extends from the origin of the STA trunk at the root of zygoma and along the course of its parietal branch. Occasionally, the frontal branch is more dominant and a curvilinear incision is used and the donor frontal branch is harvested from underneath the scalp flap. A generous piece of the artery should be targeted as stretched or twisted STAs carry a significant risk of occlusion.
Harvesting the STA Branches(s)
STA harvesting should be performed under magnification of the microscope or surgical loups to avoid injurious dissection, which could render the vessel not amenable for use. I prefer to use the microscope to ensure the availability of a healthy STA for later revascularization. The use of microscope is more ergonomic on my neck compared to the loups.
The incision is preferably commenced at the origin of the STA at the level of the zygoma and extended toward the superior temporal line along the course of the parietal branch using sharp dissection. However this dissection is technically easier if performed visa versa and I therefore, start the incision at its distal end and work toward the zygoma.
Early in dissection, the surgeon must take care not to incise deeply to avoid inadvertent injury to the vessel. Maintaining a connective cuff of tissue around the STA is key in avoiding subsequent vasospasm. The surrounding collaterals are carefully isolated, coagulated, and divided. Their avulsion injury must be avoided.
Figure 4: The initial incision may be conducted more distally along the length of the STA and carried proximally. Before the parietal branch is identified, the surgeon must take care not to incise too deeply to avoid injuring the STA and its branches within their subcutaneous course. After distal identification of the vessel, Mosquito clamps are used to gently and bluntly dissect over the vessel. Next, the clamp is used to elevate the superficial soft tissue layer away from the vessel while a knife is used to cut the superficial tissues. These steps are repeated until the vessel is dissected close to the zygomatic arch.
Figure 5: Vessel loops are used to handle the artery; its direct manipulation is avoided to prevent vasospasm. The length of the STA donor vessel may be slightly increased via further dissection around the tortuous STA trunk at the root of zygoma. Sutures are used to mobilize and retract the scalp layers (left). Note that the soft tissues immediately around the STA are left intact to protect the vessel (right image).
In case of a minor injury to the vessel during dissection, blind coagulation of the wall should be avoided. I identify the exact site of the bleeding and tamponade it using a small piece of cotton followed by precise coagulation of the bleeding site. In the case of a sizable defect in the STA’s lumen, interrupted 10.0 sutures may be used to repair the defect. The frontal branch is not to be sacrificed and may be dissected as well, as an alternative choice, or for use in case of double-barrel bypass.
The superior surface of the vessel should be marked to avoid its twisting during its mobilization and anastomosis. After the harvest is complete, the vessel and connective cuff are left intact or in continuity until it is time for anastomosis. The vessel is not usually disconnected distally until I am ready to perform the anastomosis. This technique provides an opportunity for an indirect bypass if an adequate cortical recipient vessel is not found.
Figure 6: I wrap the length of the vessel within a thin piece of allograft dura or surgical glove (left). Next, retention sutures in the wrapping material mobilize the vessel away from my working zone during performance of the muscle dissection and craniotomy (right).
After retracting the vessel aside, a vertical incision is made into the temporalis muscle in line with the long axis of the STA, which is also mobilized to expose the temporal bone. Another incision within the muscle just inferior to the superior temporal line allows adequate mobilization of the muscle. The assistant protects the STA with a wide retractor blade.
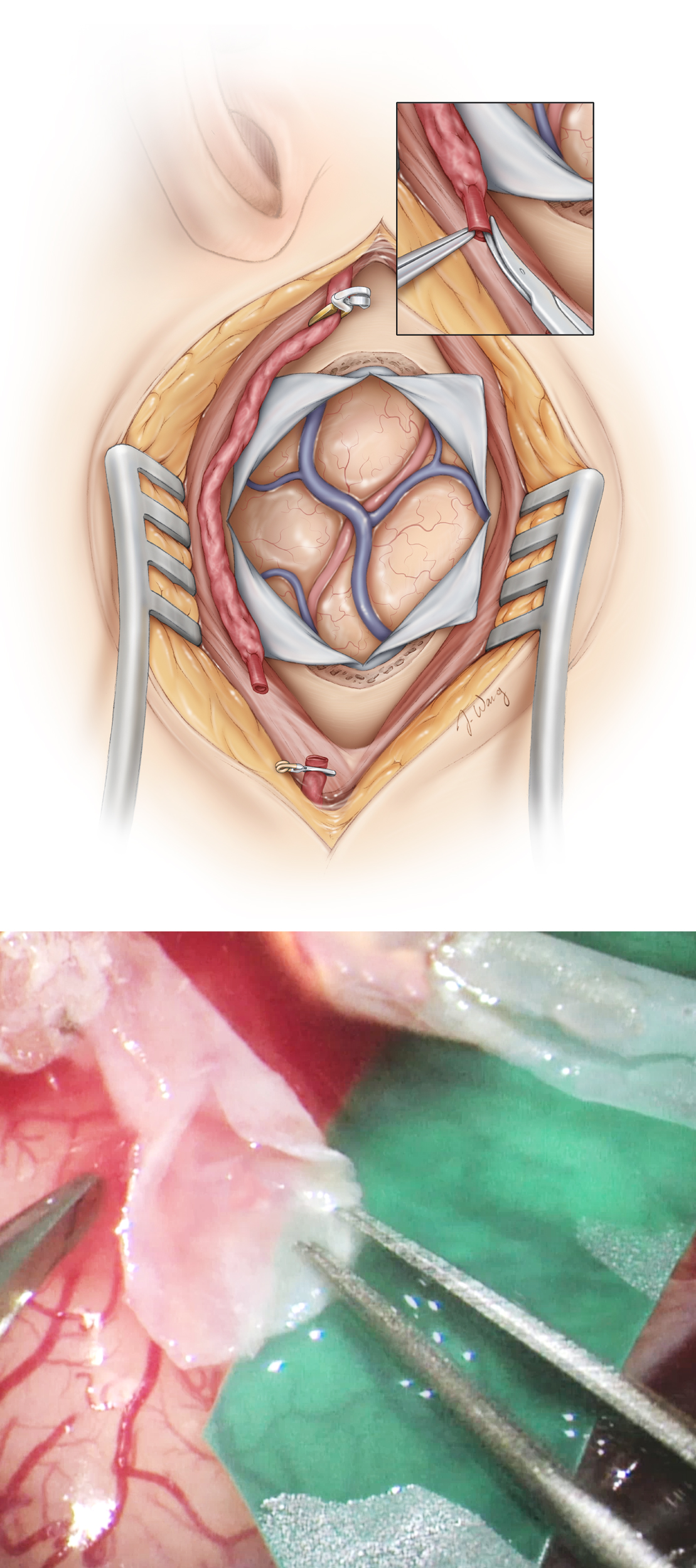
Next, a craniotomy flap, 6cm in diameter, centered over the squamosal suture and exposing the Sylvian fissure is elevated.
Dissection of the Recipient Artery
A stellate dural opening provides adequate cortical exposure. A vertical dural incision is made at the root of the harvested STA to allow its transdural tunneling during closure. Immaculate hemostasis at every step is critical to avoid run-in of blood during the anastomosis.
In patients with Moyamoya disease, care must be taken to preserve the indispensible meningo-cortical feeding vessels during the dural opening. These vessels may or may not be seen on preoperative external carotid artery angiography. At this stage, brain relaxation may be achieved by opening the regional arachnoid membranes and the consequent CSF release. Wide arachnoid dissection over the sulci is rewarding to Moyamoya patients in enhancing postoperative neovascularization.
The proper choice of a cortical MCA branch for anastomosis is critically important. The selection criteria for this branch include its accessibility, caliber, as well as its apparent proximal and distal vascular beds. In most cases, an M3 or M4 branch of about 0.9 mm or more in diameter is ideal. In case of a large STA or total replacement of MCA flow (high-flow bypass), an M2 branch may be the proper recipient choice. White and flattened arteries at areas of previous ischemic events and encephalomalacia should be avoided.
I prefer to find the recipient vessel that is perpendicular to the Sylvian fissure as this working angle promotes easy suturing. The location of the recipient vessel should allow the distal end of the donor vessel to reach for the anastomosis with some redundancy that can promote indirect revascularization.
Once a suitable branch is identified, arachnoid adherences around the vessel are released. During any coagulation process, the electrocautery power should be reduced to avoid injury to the recipient cortical branch. I apply liberal amounts of papaverine solution to the field to avoid or relieve vasospasm.
The length of the donor STA branch is measured with a ruler to ensure an appropriate length of the vessel for a tension-free anastomosis. If the STA branch appears too short, liberal dissection at the root of the STA will add an slight extension to the vessel.
Not only should anastomosis under tension be avoided, but a redundantly long vessel is also unwanted, as it may promote twisting and disruption of flow after the anastomosis is complete. Finally, a colored rubber dam is placed underneath the recipient vessel to aid with visualization of an almost transparent empty artery at the time of the anastomosis. It may be necessary to sacrifice one or two small perforating arteries originating from the dissected segment of the recipient vessel to allow its mobilization over the rubber dam.
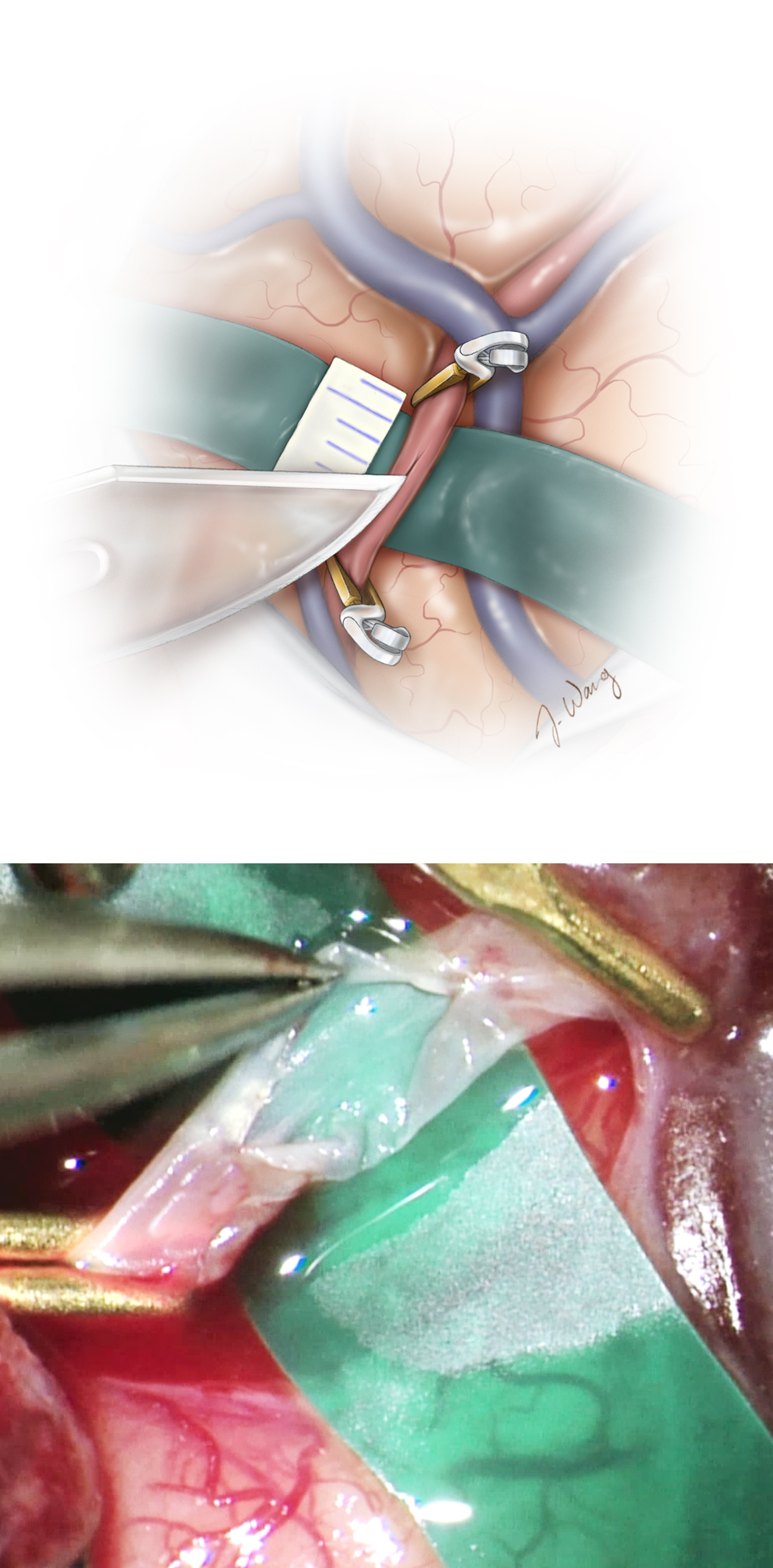
Figure 7: After the craniotomy is performed and a suitable MCA recipient branch is identified, the proximal STA is clamped using a temporary clip, and the its distal end is divided and prepared for anastomosis as shown by removing the connective tissue off its terminal 5–10 mm end. The distal end of the donor vessel is cut obliquely or “fish mouthed,” allowing for an increased anastomotic surface
Anastomosis
The aneurysm clip on the proximal STA is released momentarily to clear its lumen of debris and the vessel is flushed with heparinized saline.
Figure 8: The cortical MCA branch is then temporarily clip occluded using two miniclips, and a diamond-shaped arteriotomy is completed using a number of different blades, including a beaver ophthalmic blade or diamond knife. This arteriotomy is then extended using microscissors to about 2–3 times the diameter of the distal end of the STA branch. A 5-mm segment of the ruler is cut and used for these measurements.
When I use the microforceps to handle the donor and recipient vessel walls, I am careful to apply traction only on the outer wall layers (adventitia) rather than the inner layers, which would result in intimal injury and possibly intimal dissection. The lumen is periodically irrigated with heparinized saline as a precaution against thromboembolic consequences of clamping.
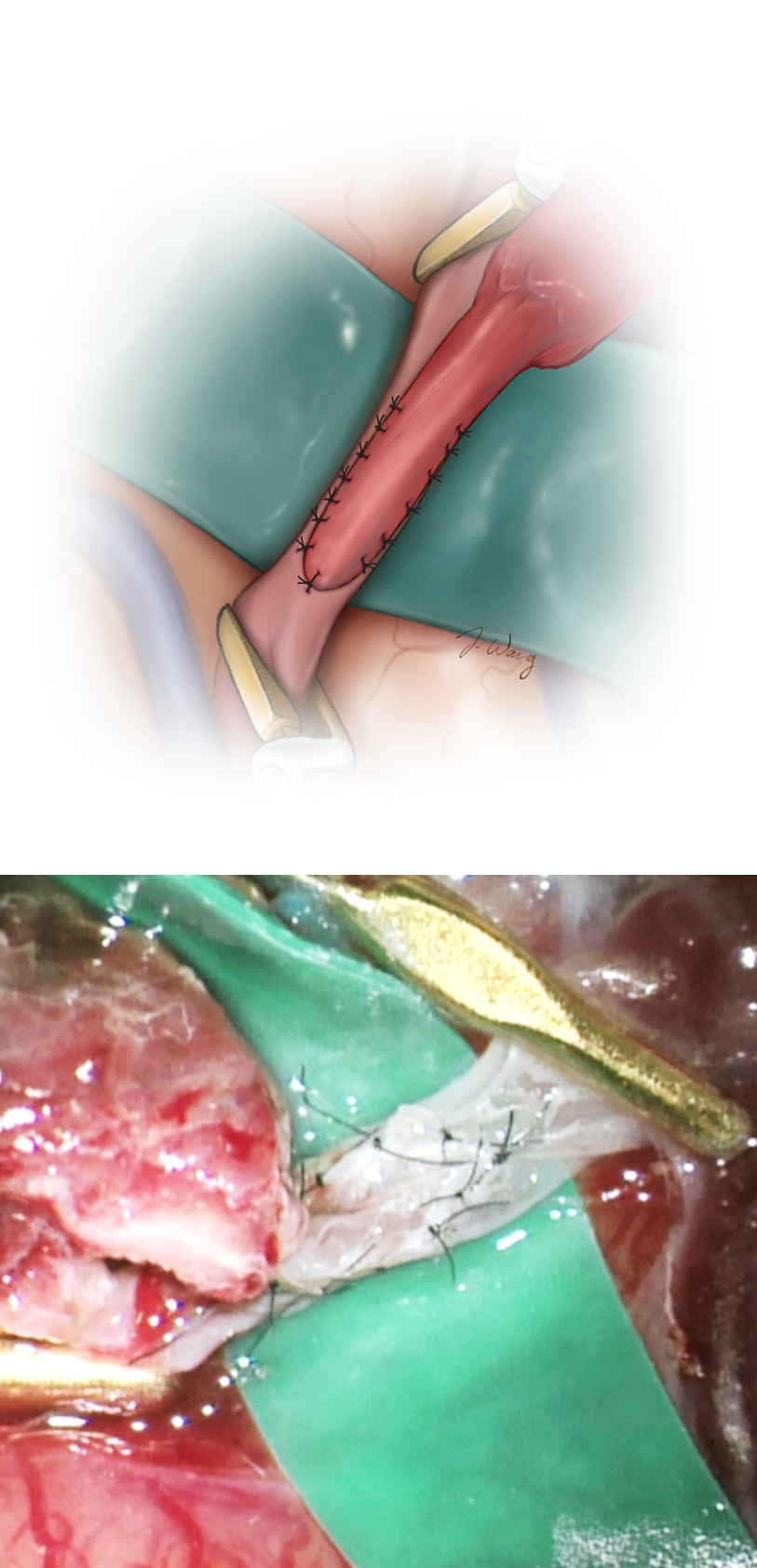
Figure 9: The initial heel and toe sutures of the anastomosis are illustrated. Using 9-0 or 10-0 nonabsorbable sutures, the heel followed by the toe of the distal end of the STA is sutured to the complementary ends of the recipient artery. The sutures are placed from an outside-to-inside direction in the STA and an inside-to-outside direction on the recipient MCA branch.
Finally, interrupted 10-0 sutures complete the end-to-side anastomosis of the STA to the MCA arteriotomy between the two initial heel and toe sutures.
The sides of the anastomosis are secured using either two running sutures or eight to ten interrupted sutures, starting with the less accessible side first. This method allows examination of the arteriotomy closure from inside the vessel lumen before closure of the opposite side. If the running suture technique is employed, the running ties are left loose until the final tie is placed and the vessel is flushed with heparinized saline; only then are the sutures tightened and the final knot created.
Figure 10: Care should be taken to suture the donor vessel from outside to inside and vice versa on the recipient vessel. If the interrupted suture technique is used, flushing is done before the final tie is placed. The needle should be turned during its passage through the walls of the vessels to avoid creation of large suture holes and tearing. Large bites of the walls of the recipient artery should not be incorporated into the suture line as this will lead to stenosis at the site of the anastomosis.
Only the needle is manipulated, and the suture is left untouched because 10-0 sutures are prone to fracture with minimal manipulation by the forceps. The needle is passed through the walls under direct vision, and blind insertion is strictly avoided because this will lead to inadvertent incorporation of both walls of the recipient vessel and its occlusion.
After completion of the anastomosis, the clips are removed in an orderly fashion, starting with the distal MCA, followed by the proximal MCA clip, and finally the proximal STA clip. Minor leaks along the anastomosis can be easily controlled with gentle tamponade using absorbable Surgicel or other hemostatic agents.
If significant leakage is evident, an additional suture may be placed after reinstallment of the clips. Before closure, the anastomosis is assessed using a microDoppler probe and/or fluorescence videoangiography. Intraoperative angiography may also be performed if the quality of the anastomosis remains in doubt.
Figure 11: I prefer the use of interrupted sutures during the STA-MCA revascularization to minimize the risk of kinking within the anastomosis, which is possible with a running-suturing technique. However, the interrupted technique requires a longer period of clamping for its execution. The donor vessel should lay on the brain surface under no tension and remain untwisted.
If graft patency is not apparent, occlusion of the anastomosis site is suspected. We reopen one side of the suture line and remove the clot in the lumen of the vessels. The donor vessel is allowed to bleed for short period. Heparin irrigation is used to flush all of the vessels and the suture line is reconstructed. If this maneuver is not effective, another recipient vessel may be selected and the procedure repeated as long as the STA is functional.
Closure
Unlike the conventional craniotomy closure, the inferior margin of the dural opening is not closed in order to avoid STA stenosis. In addition, for patients with Moyamoya disease, the dural flap may be reversed and replaced by a periosteal or muscular flap to enhance neovascularization.
Similarly, the bone flap is trimmed at its inferior margin, and the temporalis muscle is not approximated near the entry site of the STA branch.
Finally, the skin closure is completed with meticulous care to avoid any injury to the underlying donor vessel. However, placement of excessively superficial sutures, which may result in skin dehiscence and wound infection, should be avoided.
Postoperative Considerations
A noncompressive head bandage is applied and the patient should avoid sleeping on the side of the anastomosis for the first few days after surgery. Careful observation in the intensive care unit for any signs of underlying vasospasm or ischemia during the first 24 to 48 hours after surgery is recommended. The STA pulse is monitored daily using a bedside transcutaneous microDoppler device.
Blood pressure should be maintained within each patient’s normotensive or slightly hypertensive level (typically 110–140 mm Hg) immediately after surgery. Aspirin is continued indefinitely. Adequate wound care is vital to avoid dehiscence and wound infection.
Figure 12: Graft patency is assessed during subsequent follow-up exams after discharge using conventional angiography or CTA. In this angiogram, the left STA-MCA bypass is patent (three-dimensional reconstruction image, right).
It is not unusual for patients with Moyamoya disease and minimal hemodynamic reserve to neurologically worsen temporarily during the postoperative period, even in the absence of any obvious imaging findings. This phenomenon reflects the remarkably tenuous perfusion status of their hemisphere, prone to minor ischemic events despite carefully monitored and slightly hypertensive intraoperative measures.
Indirect Bypass
Some patients do not have donor and recipient vessels of appropriate size for direct revascularization. In addition, some patients have gone previous revascularization procedures and therefore do not harbor suitable vessels.
In these circumstances, I employ indirect revascularization techniques including encephaloduroarteriosynangiosis (EDAS), encephalomyosynangiosis, pericranium onlay and pedicled omental transposition.
Delayed neovascularization leads to impressive blood flow to the hypoperfused hemispheres. If EDAS is not feasible, onlay of the temporalis muscle and pericranium are reasonable alternatives.
Encephaloduroarteriosynangiosis
The initial steps of the operation regarding the STA harvest and exposure are the same as the direct bypass procedure discussed above. The STA is not disconnected and a wide soft tissue cuff is maintained along the STA.
After the dura is incised, the arachnoid layers covering the sulci including the sylvian fissure are widely opened. Next, the STA and soft tissue cuff are placed over the exposed brain surface. Stripping the arachnoid bands promotes ingrowth of neovascularization from the STA and soft tissue cuff.
Some colleagues tack the STA cuff to the underlying brain using 10-0 pial sutures to maintain direct tissue apposition. Prior to replacement of the bone, the flap should be contoured to allow unimpeded entry and exit of the STA.
Encephaloduroarteriomyosynangiosis (EDAS)
Developing Technology for Revascularization Procedures
A number of new technologies have recently been introduced with the goal of making the process of microvascular anastomosis less technically demanding.
Although none of these technologies has yet been able to replace microsurgical suture anastomosis, they have recently displayed great potential and promise. Fibrin sealants can potentially be used to perform sutureless anastomoses. These sealants may function as multiple sutures within the anastomosis line, allowing accessibility to difficult sites.
Another option is suture clips, which greatly resemble skin-closure staples and are available in two forms; nonpenetrating titanium clips and self-closing U-clips.
In an attempt to avoid the adverse effects of temporary vessel occlusion, the excimer laser-assisted nonocclusive anastomosis (ELANA) technique has been designed. An arteriotomy is formed by passing an excimer laser probe through the lumen of the recipient vessel after attachment of the donor vessel.
These new technologies, although very valuable in facilitating more ideal cerebrovascular anastomoses, have yet to overcome a number of limitations for optimal use in neurosurgery, including adequate miniaturization.
Contributors: Ahmed Enan Helal, MD, Ulas Cikla, MD and Mustafa K. Başkaya, MD
References
Arias EJ, Derdeyn CP, Dacey RG, Zipfel GJ. Advances and surgical considerations in the treatment of Moyamoya disease. Neurosurgery. 2014; 74(Suppl 1):S116-125.
Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal “misery perfusion syndrome” by extracranial-intracranial arterial bypass in hemodynamic cerebral ischemia. Stroke. 1981; 12: 454-459.
Derdeyn C, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, Powers WJ. Variability of cerebral blood volume and oxygen extraction: Stages of cerebral hemodynamic impairment revisited. Brain. 2002; 125: 595-607.
Duckworth EAM, Rao VY, Patel AJ. Double-barrel bypass for cerebral ischemia: technique, rationale, and preliminary experience with 10 consecutive cases. Neurosurgery. 2013; 73: (1 Suppl Operative):ons30-38.
EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Eng J Med. 1985; 313: 1191-1200.
Garrett MC, Komotar RJ, Merkow MB, Starke RM, Otten ML, Connolly ES. The Extracranial-Intracranial Bypass Trial: Implications for future investigations. Neurosurg Focus. 2008; 24: E4.
Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Connolly ES. Radiographic and clinical predictors of hemodynamic insufficiency in patients with athero-occlusive disease. J Stroke Cerebrovasc Dis. 2008;17; 340-343.
Gross BA, Du R. How I do it: STA-MCA bypass. Acta Neurochir. 2012: 154:1463-1467.
Hob BL, Cheung, AC, Rabinov JD, Pryor JC, Carter BS, Ogilvy CS. Results of prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery. 2004;54: 1329-1342.
Kawashima M, Rhoton A, Tanriover N, Ulm AJ, Yasuda A, Fujii K. Microsurgical anatomy of cerebral revascularization. Part I: Anterior circulation. J Neurosurg. 2005;102: 116-131.
Kuroda S. AMORE Study Group. Asymptomatic Moyamoya disease: Literature review and ongoing AMORE Study. Neurol Med Chir (Tokyo). 2015: 55:194-198.
Kuroda S, Kawabori M, Hirata K, Shiga T, Kashiwazaki D, Houkin K, Tamaki N. Clinical significance of STA-MCA double anastomosis for hemodynamic compromise in post-JET/COSS era. Acta Neurochir. 2014; 156: 77-83.
Lee S-B, Huh P-W, Kim D-S, Yoo- D-S, Lee T-G, Cho K-S. Early superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke. Clin Neurol Neurosurg. 2013;115: 1238-1244.
Li Y, Cikla U, Baggott C, Yilmaz T, Chao C, Baskaya MK. Surgical treatment of adult Moyamoya disease with combined STA-MCA bypass and EDAS: Demonstration of technique in video presentation. Turk Neurosurg. 2015; 25:126-131.
Miyamoto S, Yashimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, Nakagawara J, Takahashi JC. Effects of extracranial-intracranial bypass for patients with hemorrhagic Moyamoya disease. Results of the Japanese Adult Moyamoya Trial. Stroke. 2014; 113:1415-1421.
Nussbaum ES, Mocco J. Cerebral Revascularization: Microsurgical and Endovascular Techniques. Thieme Medical Publishers: 2011.
Ogasawara K, Ogawa A. [JET Study (Japanese EC-IC Bypass Trial)]. Nihon Rinsho. Japanese J Clin Med. 2006; 64(Suppl 7): 524-527
Powers WJ, Clarke WR, Grubb RL, Videen TO, Adams HP, Derdeyn CP. Extracranial-intracraial bypass surgery for stroke prevention in hemodynamic cerebral ischemia. The Carotid Occlusion Surgery Study Randomized Trial. JAMA. 2011;306: 1983-1992.
Sekhar L, Kalavakonda C. Cerebral revascularization for aneurysms and tumors. Neurosurgery. 2002;50: 321-331.
Sekhar L, Natarajan SK, Ellenbogen RG, Ghodke B. Cerebral revascularization for ischemia, aneurysms, and cranial base tumors. Neurosurgery. 2008;(6 Supp 3): 1373-1410.
Surdell D, Hage ZA, Eddleman C, Gupta DK, Bendok BR, Batjer HH. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008; 24:1-15
Thines L, Durand A, Penchet G, Proust F, Lenci H, Debailleul A, Lejeune J-P, Pelissou-Guyotat I. Microsurgical neurovascular anastomosis: The example of superficial temporal artery to middle cerebral artery bypass. Technical principles. Neurochirurgie. 2014; 60: 158-164.
Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: Techniques and applications in the evaluation of chronic cerebral ischemia. AJNR. 2009; 30: 876-880.
Vilela MD, Newell DW. Superficial temporal artery to middle cerebral artery bypass: past, present, and future. Neurosurg Focus. 2008;24: 1-9.
Wintermark M, Sesay M, Emmanuel B, Borbély K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza SP, Soustiel J-F, Nariai T, Zaharchuk G, Caillé J-M, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005; 36: 83-99.
Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Nakamura K, Yamamoto Y, Yonekura Y, Konishi J, Kimura J. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. 1996;61:18-25.
Yasargil MG. Personal considerations on the history of microneurosurgery. J Neurosurg 2010;112; 1163-1175.
Please login to post a comment.