Brainstem AVMs
This is a preview. Check to see if you have access to the full video. Check access
Anterior Pontine AVM and Meckel's Cave dAVF: The "in situ" Occlusion Technique
Please note the relevant information for patients suffering from arteriovenous malformations is presented in another chapter. Please click here for patient-related content.
Operative Anatomy
The brainstem consists of the midbrain, pons, medulla, and superior, middle, and inferior cerebellar peduncles. It is separated from the cerebellum by the cerebellomesencephalic, cerebellopontine, and cerebellomedullary fissures.
The posterior circulation includes the relevant vascular pedicles for brainstem arteriovenous malformations (AVMs). The basilar artery serves as the major vascular supply for the brainstem, with three main branches that include the superior cerebellar artery (SCA), the anterior inferior cerebellar artery (AICA), and the posterior inferior cerebellar artery (PICA).
The SCA comprises four segments:
- Anterior pontomesencephalic segment (S1): from the SCA origin to the anterolateral brainstem coursing beneath cranial nerve (CN) III
- Lateral pontomesencephalic segment (S2): from the anterolateral brainstem caudally to the trigeminal root until it enters the cerebellomesencephalic fissure
- Cerebellomesencephalic segment (S3): within the cerebellomesencephalic fissure and eventually accompanying CN IV through hairpin curves and reaching the tentorial edge
- Cortical segment (S4): emerges from the cerebellomesencephalic fissure and supplies the tentorial surface of the cerebellum
Click here to view the interactive module and related content for this image.
Figure 1: The relevant segmental anatomy of the SCA is shown (images courtesy of AL Rhoton, Jr).
The AICA also comprises four segments:
- Anterior pontine segment (A1): from the origin of the AICA, proximal to CN VI root exit zone, and ending inferiorly along the long axis of the inferior olive
- Lateral pontine segment (A2): from the anterolateral margin of the pons through the cerebellopontine angle (CPA) up to the flocculus, with terminal branches including: the labyrinthine artery, recurrent perforating artery, and subarcuate artery
- Flocculopeduncular segment (A3): from the flocculus superiorly to the cerebellopontine fissure, commonly with rostral and caudal trunks surrounding CNs VII/VIII
- Cortical segment (A4): distal to the cerebellopontine fissure to supply the cerebellar petrosal surface
Click here to view the interactive module and related content for this image.
Figure 2: The relevant segmental anatomy of the AICA is shown (images courtesy of AL Rhoton, Jr).
The PICA is divided into five segments:
- Anterior medullary segment (P1): the origin of the PICA courses anterior to the medulla and passes along the hypoglossal rootlets, terminating at the medial border of the inferior olive
- Lateral medullary segment (P2): this short segment has its origin at the most prominent point of the inferior olive to the rootlets of CNs IX/X/XI at the lateral edge of the olive
- Tonsillomedullary segment (P3): descends from the lateral edge of the olive to the inferior pole of the cerebellar tonsil and reverses rostrally along the medial tonsil to its midpoint (the infratonsillar/caudal loop)
- Telovelotonsillar segment (P4): ascends from the midpoint of the cerebellar tonsil toward the roof of the fourth ventricle and turns caudally, coursing posteriorly toward the tonsillobiventral fissure (the supratonsillar/rostral loop). This branch perfuses the choroid plexus of the fourth ventricle and the inferior medullary velum.
- Cortical segment (P5): its origin emerges from the tonsillobiventral fissure with two medial and lateral trunks for the vermis and tonsils/cerebellar hemisphere, respectively
Click here to view the interactive module and related content for this image.
Figure 3: The relevant segmental anatomy of the PICA is shown (images courtesy of AL Rhoton, Jr).
The segmental anatomy of the brainstem, with the corresponding cerebellar peduncle, major fissure, and arterial feeder, allows for a discussion of the anatomy based on three neurovascular complexes:
- Upper complex: midbrain, cerebellomesencephalic fissure, superior cerebellar peduncle, tentorial cerebellar surface, the SCA, and CNs III, IV, and V
- Middle complex: pons, cerebellopontine fissure, middle cerebellar peduncle, cerebellar petrosal surface, the AICA, and CNs VI, VII, and VIII
- Inferior complex: medulla, cerebellomedullary fissure, inferior cerebellar peduncle, suboccipital cerebellar surface, the PICA, and CNs IX, X, XI, and XII
These divisions also have evolutionary and developmental significance and relationships.
Designation of brainstem veins is not as straightforward as the arterial anatomy because venous appellation is based on the direction of venous drainage (longitudinal or transverse) and the adjacent segment and surface of the brainstem. The longitudinal veins and the posterior communicating, tectal, and peduncular veins drain into the basal vein of Rosenthal en route to the vein of Galen. Anterior brainstem veins, which are most often transverse, drain into the superior petrosal and inferior petrosal veins en route to the petrosal sinuses.
The complexity of the neurovascular anatomy of the brainstem demands that the surgeon has an excellent intraoperative anatomic awareness. The combination of precise knowledge of the anatomy, specific brainstem landmarks, and three-dimensional conceptualization of their relationship is the mainstay for any surgical approach to the brainstem, particularly for AVMs.
The close proximity of the major motor, sensory, and autonomic tracts, in addition to the cranial nerves and their corresponding nuclei, highlights the importance of identifying the landmark anatomic points reliably. Following is a brief discussion of the pertinent landmarks for a rapid review:
- CN III exits from the anterior midline in the pontomesencephalic fissure and courses between the PCA and SCA.
- CN IV emerges from the dorsal brainstem surface in the cerebellomesencephalic fissure below the inferior colliculus, and wraps around the midbrain adjacent and inferior to the tentorial edge.
- CN V emerges from the posterolateral aspect of the pons, coursing anteriorly through the cerebellopontine angle cistern.
- CN VI exits anteriorly in the pontomedullary fissure rostral to the anterolateral sulcus near the origin of the AICA and courses in the prepontine cistern to the Dorello’s canal.
- CNs VII and VIII emerge posterolateral to the pontomesencephalic fissure and course laterally in the cerebellopontine angle cistern toward the internal acoustic meatus.
- CNs IX, X, and XI arise from the posterolateral sulcus of the medulla and course laterally to the jugular foramen within the cerebellomedullary cistern.
- CN XII emerges from the anterolateral sulcus of the medulla into the premedullary cistern and courses laterally within the cerebellomedullary cistern and the hypoglossal canal.
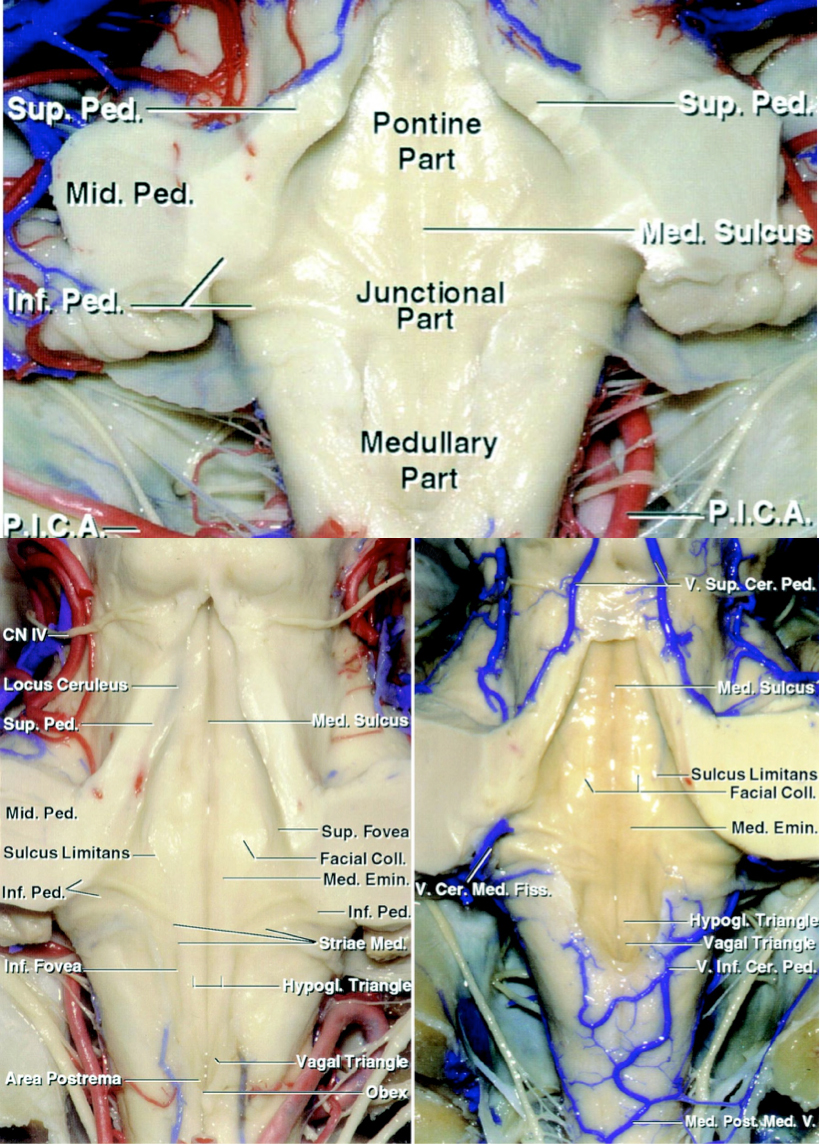
- The fourth ventricular floor has a configuration that resembles a rhomboid containing pontine, junctional, and medullary regions. These regions are detailed below.
Click here to view the interactive module and related content for this image.
Figure 4: The surface anatomy of the fourth ventricle and its landmarks are shown. Please refer to the text for more details.
The fourth ventricular floor contains the following anatomic landmarks:
- Pontine part is triangular, with the apex at the cerebral aqueduct. Its lateral limbs are the medial borders of the superior cerebellar peduncles, and the base is an imaginary line connecting the lower margin of the cerebellar peduncles.
- Junctional part is the strip between the lower margin of the cerebellar peduncles and the tela choroidea attachment to the teniae just below the lateral recess.
- Medullary part is also triangular shaped and is limited laterally by the teniae and the obex at the caudal tip, anterior to the foramen of Magendie.
- Median sulcus divides the fourth ventricular floor into rostral-to-caudal symmetrical halves.
- Sulcus limitans is another parallel longitudinal sulcus lateral to the median sulcus bilaterally.
- Median eminence is the raised strip along the fourth ventricular floor between the median sulcus and sulcus limitans containing the facial colliculus (a rounded prominence overlies the nucleus of the abducens nerve and the ascending section of the root of the facial nerve).
- Three triangular areas overlie the hypoglossal nucleus, vagus nucleus, and area postrema.
- Locus ceruleus is a bluish gray area (pigmented due to melanin granules) located at the rostral end of each sulcus limitans in the lateral margin of the fourth ventricular floor.
- Vestibular area is located lateral to the sulcus limitans along the fourth ventricular floor, and just deep to this region is the vestibular nuclei.
- Auditory tubercle is a prominence in the lateral part of the vestibular area formed by the underlying dorsal cochlear nucleus and the cochlear part of the vestibulocochlear nerve.
Figure 5: The basic anatomy of the brainstem and associated vasculature is shown (image courtesy of AL Rhoton, Jr).
BRAINSTEM ARTERIOVENOUS MALFORMATION SUBTYPES
Brainstem AVMs are rare and comprise approximately 5% of all brain AVMs. According to the location and eloquence of the brainstem, all brainstem AVMs are associated with moderate to high surgical risk. The intrinsically exquisite eloquence of the brainstem demands no transgression of the brainstem parenchyma or pia during resection of the lesion.
The pial dissection may be strictly limited by “in situ” occlusion. The arterial feeders are reliably recognized and disconnected, after which the draining vein turns dark blue. Subsequently, the vein(s) is disconnected, but the nidus is left in the brainstem and is considered nonfunctional. Some surgeons advocate the “pial resection technique.” In other words, only the extraparenchymal portion of the AVM and its associated feeders and draining veins are resected, and the intrinsic portions are left untouched.
Brainstem AVMs may have epipial, pial, or subpial (parenchymal) involvement, but usually they are pial or epipial, differentiating themselves from cerebral or cerebellar AVMs. In a patient with a ruptured AVM, the hematoma may create nonanatomic planes and allow transgression of the brainstem. In this setting, the surgeon can resect the entire nidus despite its parenchymal involvement.
One of the most important surgical nuances in brainstem AVM surgery is preservation of the normal perforating branches. They are small in caliber and difficult to differentiate from AVM feeders in the restricted and crowded surgical field. The eloquence of the distal territories makes manipulation of these vessels high risk. Even minor damage to a perforator or its vasospasm can lead to severe neurologic complications. Therefore, the expertise and compulsion of the operator play a paramount role in the patient’s outcome.
Neurophysiologic monitoring, including brainstem auditory evoked responses (BAERs), as well as somatosensory (SSEPs) and motor evoked potentials (MEPs), are employed during almost all operative interventions related to brainstem AVMs.
The following sketches describe the general principles for selection of the appropriate approach for brainstem lesions.
Figure 6: The corresponding craniotomies for reaching brainstem lesions are demonstrated. Suboccipital, retrosigmoid, supracerebellar and orbitozygomatic approaches are favored for most lesions.
Anterior Midbrain AVMs
These lesions reside on the anterior midbrain surface and often involve the cerebral peduncles. They are particularly high risk to dissect because of their close proximity to CN III and mesencephalic perforating vessels. The AVM feeders are derived from the P1 and basilar bifurcation perforators that also supply the posterior thalamus and cerebral peduncles. The venous drainage ultimately joins the basal vein of Rosenthal, which should be identified at the lateral margins of the lesion.
An orbitozygomatic or pterional craniotomy, similar to the approach used for basilar apex aneurysms, is recommended. Following generous Sylvian fissure and arachnoid dissection, the Liliequist’s membrane is divided and the intrapeduncular cistern is entered. More specifically, I pursue the posterior communicating artery and dissect the Liliequist’s membrane toward the interpeduncular cistern. This approach allows sufficient surgical exposure along the anterior midbrain and good basal viewing angles.
The potential operative corridors are the opticocarotid triangle, supracarotid triangle, and the triangle between CN III and the tentorium. A combination of corridors should be optimally selected according to the location and size of the lesion. For further details, please refer to the Basilar Bifurcation Aneurysm: Pterional Approach chapter.
As the first step, I identify the mesencephalic veins at the lateral margins of the AVM. Next, it is essential to differentiate the PCA AVM feeders from normal perforators at the AVM margins. Occlusion of the AVM feeders is conducted anteriorly, followed by circumdissection limited to the pial surface. I do not transgress the pia into the subpial structures. Division of the feeders at the nidus leads to darkening of the draining veins. Finally, I decide to either resect the epipial nidus or occlude it in situ based on the extent of its invasion within the pia mater/parenchyma.
While performing the critical steps around the nidus, it is imperative to be mindful of the integrity of CN III, the cerebral peduncles, and the normal perforators. Therefore, the extent of bipolar coagulation should be minimized to avoid thermal injury to these structures. Following complete occlusion of the AVM feeders and its circumdissection, the use of fluorescence videoangiography is beneficial to confirm the lack of residual arteriovenous shunting. The temporary occlusion test of the vein, as described previously in the Nuances of AVM Resection chapter, is also effective to evaluate any remnant shunting.
Figure 7: Naturally, this is a deep-seated AVM with many vital structures in the proximity (top image.) A wide operative corridor is mandatory via generous Sylvian fissure dissection. The typical angioarchitecture of an anterior midbrain AVM is demonstrated. Please note the deep perforating arteries within the brainstem that have to be strictly protected. The pia must not be violated during resective maneuvers (bottom image.)
Posterior Midbrain AVMs
These lesions are located on the tectum or quadrigeminal plate between the pineal gland and the superior cerebellar peduncles. AVM surgery in this location has a high likelihood of injuring CN IV at its exit zone.
Posterior midbrain AVMs are primarily fed by the circumflex perforators from the P1 and P2 segments at their lateral front and by the S3 SCA segments at their posteroinferior borders. Venous drainage is primarily via the vein of cerebellomesencephalic fissure and the tectal vein; both ascend toward the vein of Galen.
The optimal surgical approach to most posterior midbrain AVMs is the paramedian supracerebellar-infratentorial approach. The patient is positioned in the sitting or lateral position. The major advantage of the sitting position is gravity retraction on the cerebellum, providing a retractorless panoramic view of the posterior upper brainstem. I have also used the lateral position effectively with no need for fixed retractors.
After the craniotomy, the dura is opened via a curvilinear incision based on the transverse sinus, and tented superiorly using two sutures in the tentorium anterior to the transverse sinus. This maneuver elevates the venous sinus. This step should be performed carefully to avoid occluding the sinus.
The next step involves additional cerebrospinal fluid (CSF) drainage through the lumbar drain, decompressing the cerebellum, and widening the supracerebellar corridor. One or two paramedian small bridging veins are sacrificed near the superior cerebellar surface. The quadrigeminal cistern is then exposed after its arachnoid bands are widely disconnected.
During the devascularization phase of the operation, the venous drainage system by the tectal and cerebellomesencephalic veins is identified along the superior or posterior aspects of the AVM. Arterial feeders from the circumferential perforators are found laterally on both sides of the nidus.
Additional feeding arteries from the SCA are most frequently encountered at the inferior aspect of the AVM. These arterial feeders are coagulated and divided, allowing me to mobilize the nidus upward and conduct a complete anterior circumdissection to isolate the darkening nidus. Depending on the extent of anterior pial invasion, I proceed to resect the nidus or perform an in situ occlusion versus an epipial nidal resection (pial resection technique).
Figure 8: A lateral (paramedian) supracerebellar route is ideal for resection of posterior midbrain AVMs. The feeding arteries laterally and inferiorly are disconnected and the AVM mobilized medially. Any unaffected or salvageable veins that connect to the major draining vein are protected. The exit zone of the IV nerve is highly at risk (top illustration.) This sagittal illustration (bottom photo) demonstrates the vascular anatomy of the feeding arteries and draining veins.
Figure 9: A nonoperative circumferential AVM encircling the midbrain is demonstrated. Note the lateral and anteroposterior vertebral artery angiograms identifying the venous drainage systems.
Anterior Pontine AVMs
Anterior Pontine AVM: Technical Challenges
Anterior pontine lesions are located on the anterolateral surface of the pons between and round the CN V root entry zone and the basilar sulcus, and between the pontomesencephalic fissure superiorly and the pontomedullary fissure inferiorly. These lesions are intimately associated with the trigeminal nerve root entry zone.
Anterior pontine AVMs are fed primarily by the S1 SCA superiorly and the A1 AICA inferiorly. The basilar trunk and rarely the meningohypophyseal trunk traveling through Meckel’s cave provide tributories. The venous drainage for these lesions is directed medially by the median anterior pontomesencephalic vein or laterally by the superior petrosal sinus.
Figure 10: An anterior pontine AVM is illustrated. Please note the relationship of the malformation to the fifth nerve near the brainstem. The operative blind spot medial to the nerve leads to additional technical challenges during the circumferential discovery of the nidus (top sketch, surgeon's view). Bleeding from the deep white matter feeders near the root entry zone of the nerve during AVM disconnection is problematic; this bleeding has to be handled with care in order to avoid neuropathic facial pain. The dissection should be strictly epipial. An axial view of the pathology is also included; the feeding vessels from the SCA are apparent (bottom illustration).
To achieve adequate exposure of the anterolateral pons, I use the extended retrosigmoid approach with the patient in the lateral position. After a limited posterior mastoidectomy, I expose the transverse-sigmoid junction and unroof the sigmoid sinus.
The dura is opened along the transverse and sigmoid sinuses and dural retraction sutures mobilize the sigmoid sinus laterally, enhancing the exposure toward the cerebellopontine angle. The corresponding cisterns are unveiled to efflux CSF and decompress the cerebellum. Next, I generously dissect the arachnoid membranes between CNs V and VII-VIII. Gentle mobilization and dynamic retraction of the cerebellum uncovers the anterolateral pons. The superior petrosal veins are carefully preserved.
To begin the devascularizing phase of the operation, I identify the lateral bridging veins (branches of the superior petrosal sinus); some of these veins do not take part in the anterior pontine AVMs. Fluorescence angiography or a temporary occlusion test is used to confirm the identity of the vein(s)(arterialized versus normal), followed by its coagulation and division, when appropriate. This maneuver serves to optimize the surgical field of view toward the anterolateral pons for occlusion of the arterial feeders.
Descending arterial feeders from the SCA are identified and occluded through the working angles provided above the trigeminal nerve (supratrigeminal triangle). Next, the ascending feeders from the AICA are identified and divided through the infratrigeminal triangle. The working space between CNs V and VII/VIII is most spacious and effective.
Circumdissection must progress through these triangles to mobilize the nidus posteriorly, facilitating visualization and surgical access medially. This step also allows identification of the feeders emerging from the basilar trunk.
The main obstacle with this approach is the presence of tethering CN V and arterialized branches of the superior petrosal sinus within the center of the surgical field. In most cases, it is impossible to completely resect the nidus, especially the portion medial to the trigeminal root entry zone. Fortunately, most of these lesions are epipial and can be removed without invasion of the parenchyma.
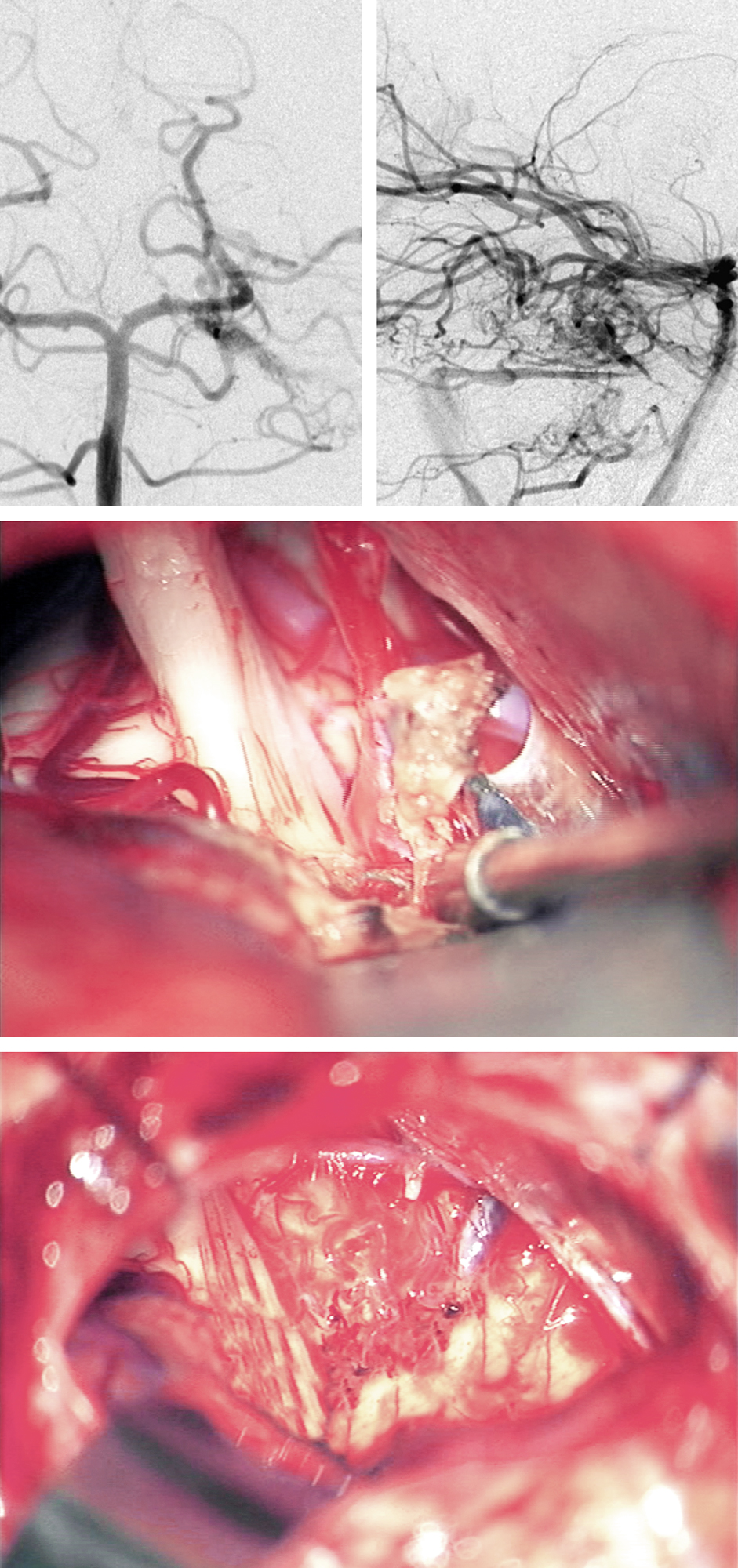
Figure 11: The anteroposterior and lateral vertebral angiograms demonstrate an anterior pontine AVM that owns a lateral venous drainage system via the superior petrosal sinus. The middle intraoperative photo demonstrates the venous drainage and the location of the CN V root entry zone. The epipial portion of the AVM nidus was removed while leaving a portion of the nidus and the disconnected feeding vessels on the pial surface of the anterior pons (inferior photo).
Anterolateral Pontine AVM
Lateral Pontine AVMs
Lateral pontine lesions are located on the lateral surface of the pons and middle cerebellar peduncle between the CN V root entry zone medially and cerebellopontine fissure laterally. This particular subtype is most likely the most common brainstem AVM.
Arterial feeding vessels arise from the A2 and A3 segments of the AICA, and like the anterior pontine AVMs, they also harbor dominant feeding vessels from the SCA. The venous drainage of these lesions is directed laterally into the superior petrosal sinus.
Figure 12: A lateral pontine AVM is demonstrated (surgeon's view, top image). Note the relationship of the lesion to the trigeminal nerve root entry zone. Compared to anterior pontine AVM, the more lateral location of the lesion improves the safety of its resection. The AICA feeding vessels are often hidden on the other side of the nidus away from the surgeon, making their control challenging. An axial sketch (bottom image) further illustrates the pathoanatomy.
Similar to anterior pontine AVMs, the lateral pontine AVMs may also be reached through the extended retrosigmoid approach. An alterative approach to the surgical exposure is an anterior transpetrosal osteotomy. I do not believe this radical skull base exposure is necessary for any of the pontine AVMs.
Following the extended retrosigmoid approach, CN V is found medial or anterior to the lesion, and therefore, the superior or inferior trigeminal triangles are not accessed. The non-arterialized branches of the superior pertrosal sinus are divided to provide medial mobilization of the hemisphere to access the peduncle.
Next, feeding vessels from the AICA are identified and disrupted inferior or anterior to the lesion between CNs V and VII-VIII. Circumdissection of the lesion and parenchymal isolation of the nidus is safe because the middle cerebellar peduncle is not eloquent. I mobilize the nidus anteriorly into the cerebellopontine angle. Lateral pontine AVMs are the most suitable brainstem subtype for complete resection with minimal neurologic complications.
Figure 13: The anteroposterior and lateral vertebral angiograms demonstrate a lateral pontine AVM that drains via the superior petrosal sinus. The second intraoperative photo demonstrates the arterialized (purple arrow) and normal branches (blue arrow) of the superior petrosal sinus as well as the nidus. The CN V root entry zone is anterior to the nidus. The third photo demonstrates the feeding arteries arising from the AICA (red arrow). After removal of the AVM and transection of the arterialized component of the superior petrosal sinus, the normal branches of this sinus were left intact (blue arrow). The en passage SCA was left unharmed (red arrow).
Lateral Pontine AVM
Anterior Medullary AVMs
Anterior medullary lesions reside caudal to the pontomedullary sulcus and anterior to the hypoglossal rootlets. These lesions often cross the midline, and fortunately they are rare compared with other brainstem AVMs.
Based on their precise location relative to the vertebrobasilar junction, the arterial feeders originate from the vertebral arteries and/or the PICA/AICA bilaterally. The venous drainage includes the median anterior medullary vein.
AVMs of the anterior medulla are generally considered inoperable unless they present with a rupture and a sizable associated hematoma. The hematoma can provide a reasonable operative trajectory by means of the trans-fourth ventricular/telovelar approach.
These lesions may also be approached through a lateral trajectory via a lateral suboccipital or far lateral transcondylar craniotomy. During the procedure, the key anatomic structures to identify and preserve include the vertebral artery, PICA, and CNs IX, X, XI, and XII. Despite the potential for an operative solution, patients with ruptured anterior medullary AVMs are often in suboptimal neurologic condition, and surgical intervention is not likely to be indicated.
Figure 14: The tributaries to an anterior medullary AVM are demonstrated. The arterial feeders originate from the vertebral arteries and/or the PICA/AICA bilaterally. The venous drainage includes the median anterior medullary vein. The hemorrhage is likely to lead to neurological decline and intervention is warranted in select cases.
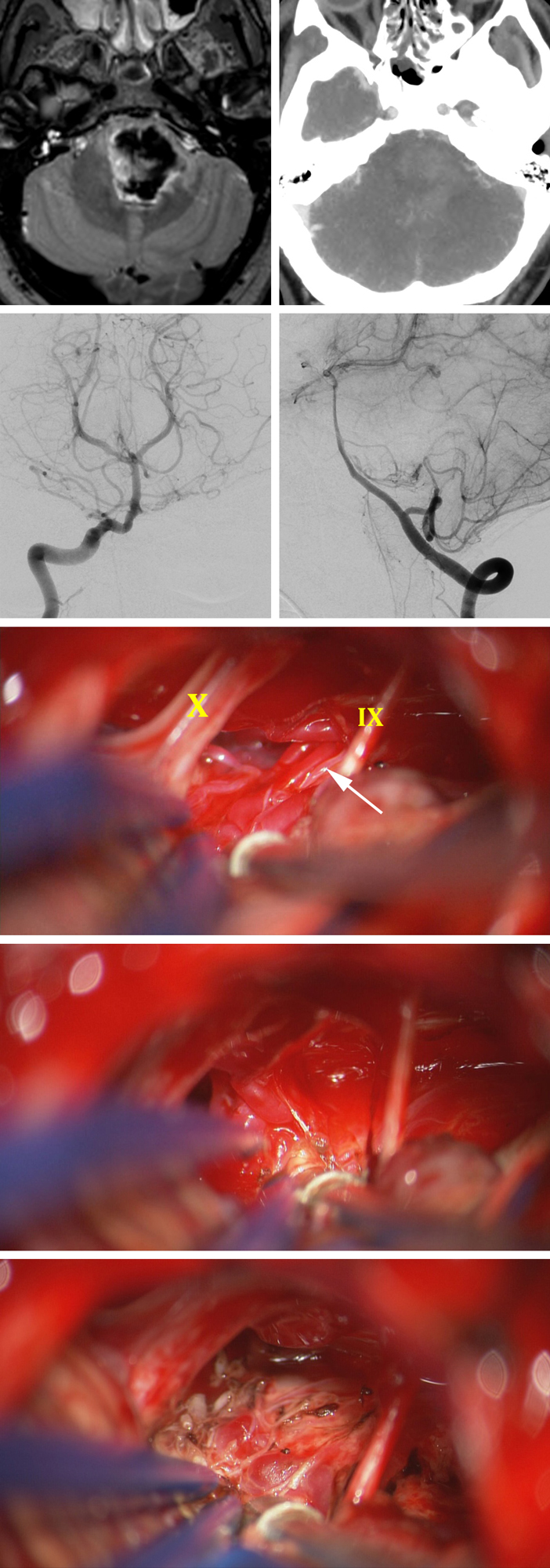
Figure 15: This unfortunate 31 year-old male presented with sudden hemiplegia and was found to suffer from a large pontine hematoma; CT angiogram was relatively unremarkable (top row). AP and lateral vertebral angiograms demonstrated a small pontomedullary AVM fed partly by the branches from the proximal basilar artery and AICA (second row). The AVM was exposed via a left-sided retrosigmoid craniotomy (third row). The nidus was disconnected via the in situ occlusion technique (fourth row). The remaining dark draining vein on the surface of the brainstem was also coagulated at the end of the operation (last row).
Anterior Pontomedullary AVM: In Situ Occlusion Technique
Lateral Medullary AVMs
Lateral medullary lesions reside on the lateral aspect of the medulla, posterior or lateral to the hypoglossal rootlets. These lesions are considered to be the most common medullary AVM.
The major feeding arterial vasculature is limited to the small branches arising from the posterolateral aspect of the vertebral artery and the P2 PICA segment. The venous drainage for these lesions is through the lateral medullary and median anterior medullary veins.
During operative intervention, I place the patient in the park-bench position with the head rotated contralaterally and the neck flexed and bent toward the floor to open the space along the ipsilateral craniocervical junction. Using a “hockey stick” scalp incision, I complete a lateral occipital craniotomy, minimal condylectomy, and C1 laminectomy. Please refer to the chapter on the transcondylar approach for more details. Neurophysiologic monitoring of the lower cranial nerves is appropriate.
The far lateral approach will optimally expose the lateral medullary AVMs. The dura is opened parallel to the skin incision, based laterally, and reflected against the condyle. The intradural surgical corridor is widened by a generous subarachnoid dissection, followed by CSF drainage. Next, the surgeon can identify the vertebral artery and its intradural entrance, permitting its skeletonization.
The cerebellomedullary fissure is dissected and the tonsil is elevated to expose all lower cranial nerve roots and intervening triangles. I design the operative channels through these triangles while staying mindful of preserving the integrity of CNs IX-XII. Next, the lateral venous drainage is identified and preserved.
The lateral and superior arterial feeders from the vertebral artery and PICA are coagulated and divided. The vertebral artery is mobilized laterally using dynamic retraction and covered by a small piece of cotton or cottonoid. Lateral medullary AVMs are predominantly pial-based without parenchymal involvement and can be completely circumdissected from the medulla. The pial resection technique is appropriate if any parenchymal invasion is present.
Figure 16: A lateral suboccipital or its far lateral variant are reasonable choices for exposure of the lesion (top photo.) I have transected 1-2 spinal accessory nerve contributions to the XI without any consequence and this maneuver is an option and often mandatory in these AVMs. These AVMs are purely epipial and amenable to resection without pial invasion. Skeletonization of the vetebral artery and vital en passage perforating arteries are paramount (top). You can further appreciate the relationship to the PICA and vertebral artery in the bottom image.
Personal Reflections
The decision-making process for brainstem AVM surgery is complex and controversial. Based on the debated efficacy of different treatment modalities and the natural history of the lesion, the patient and a complete multidisciplinary team of clinicians should be involved in the decision-making process.
The most important factor for improving the natural course of brainstem AVMs, regardless of the choice of treatment modality (microsurgery or radiosurgery,) is the expertise of the treating team and the surgeon.
Contributors: Benjamin K. Hendricks, MD, and Rouzbeh Shams-Amiri, MD
References
Lawton, MT. Seven AVMs: Tenets and Techniques for Resection. New York, Stuttgart: Thieme Medical Publishers, 2014.
Please login to post a comment.