Basilar Aneurysm: Pterional Approach
This is a preview. Check to see if you have access to the full video. Check access
Clip Ligation of a Basilar Artery Bifurcation Aneurysm
Aneurysms of the basilar artery pose some of the greatest technical challenges to both microsurgical and endovascular neurosurgeon. The length of the basilar artery and its bifurcation are lined with indispensable perforating arteries.
Distal basilar artery aneurysms (including the basilar bifurcation and superior cerebellar artery aneurysms) comprise 65% of all posterior circulation aneurysms and 7% of all cerebral aneurysms.
Although surrounded by numerous unforgiving perforators, these aneurysms harbor a more consistent projection than other aneurysms, most often projecting anteriorly and superiorly. Occasionally these aneurysms grow posteriorly into the interpeduncular cistern.
If they reach a giant size, they can impact the midbrain and third ventricle. Subarachnoid hemorrhage secondary to basilar bifurcation aneurysms leads to poor outcomes; these aneurysms lead to prehospital mortality rates three times greater than those of anterior circulation aneurysms. They also cause worse clinical grade on admission.
Indications for Microsurgery
Given the high risk of clip ligation associated with these aneurysms, most basilar bifurcation aneurysms are currently managed via endovascular techniques. Operative indications for microsurgical clipping include contraindications to endovascular therapy in difficult-access cases related to bilateral vertebral artery stenosis or tortuous vascular anatomy, large neck-to-dome ratio in young patients, or presence of a nonfetal P1 branch origin integrated into the aneurysm neck.
Other features favorable for microsurgical ligation are the presence of a noncontiguous aneurysm thought to be more amenable to clipping than coiling; the rare presence of a compressive hematoma requiring evacuation; or the relative location of the aneurysm within 5 mm of the posterior clinoid, permitting surgical access through the pterional corridor.
Other factors influencing the need for treatment and its modality include the patient’s age and general medical condition; aneurysm size, location, and projection; intraluminal thrombus; calcification; presenting symptoms; and the patient’s wishes and the experience of the practitioner. Calcified and posterior-projecting aneurysms are best suited for endovascular therapy.
Development of acute cranial nerve dysfunction related to the aneurysm requires urgent treatment of the aneurysm.
Preoperative Considerations
As discussed previously, most aneurysms selected for microsurgical intervention have a broad neck that engulfs the origin of the P1 branches. A cerebral arteriogram with three-dimensional reconstruction sequences is useful for defining the morphology of the neck, projection of the dome, and anatomy of the surrounding vasculature, including the dominance of the posterior communicating artery and P1 segments. It is imperative to derive as much information from the preoperative images as possible for safe placement of the clip.
An aneurysm neck within 5 mm of the posterior clinoid can be handled via a pterional craniotomy. Lesions located more inferiorly are exposed via the subtemporal approach. Basilar trunk aneurysms are most suited for endovascular treatment.
The height of each shoulder of P1 relative to the neck of the aneurysm will affect the side of approach and the angle of clip deployment.
Figure 1: A typical basilar bifurcation artery aneurysm is demonstrated (upper row). This aneurysm underwent microsurgical clip ligation because of the young age of the patient and favorable anatomy of the aneurysm. The altitude of the neck relative to the dorsum sella has important implications in operative planning (bottom image).
Operative Anatomy
The basilar artery ends at its quadrification within the interpeduncular fossa, and it divides into two posterior cerebral and two superior cerebellar arteries. The P1 segment of the posterior cerebral artery (PCA) begins at this quadrification and ends at the junction of the posterior communicating artery and the PCA where the P2 segment begins. The posterior communicating artery gives rise to the anterior thalamoperforating arteries along its superior and lateral aspects. These perforators supply the posterior hypothalamus, anterior thalamus, and posterior limb of the internal capsule.
The calibers of the posterior communicating artery and the P1 branch have a reciprocal relationship. A dominant or fetal posterior communicating artery is associated with a hypoplastic or absent P1 and vice versa.
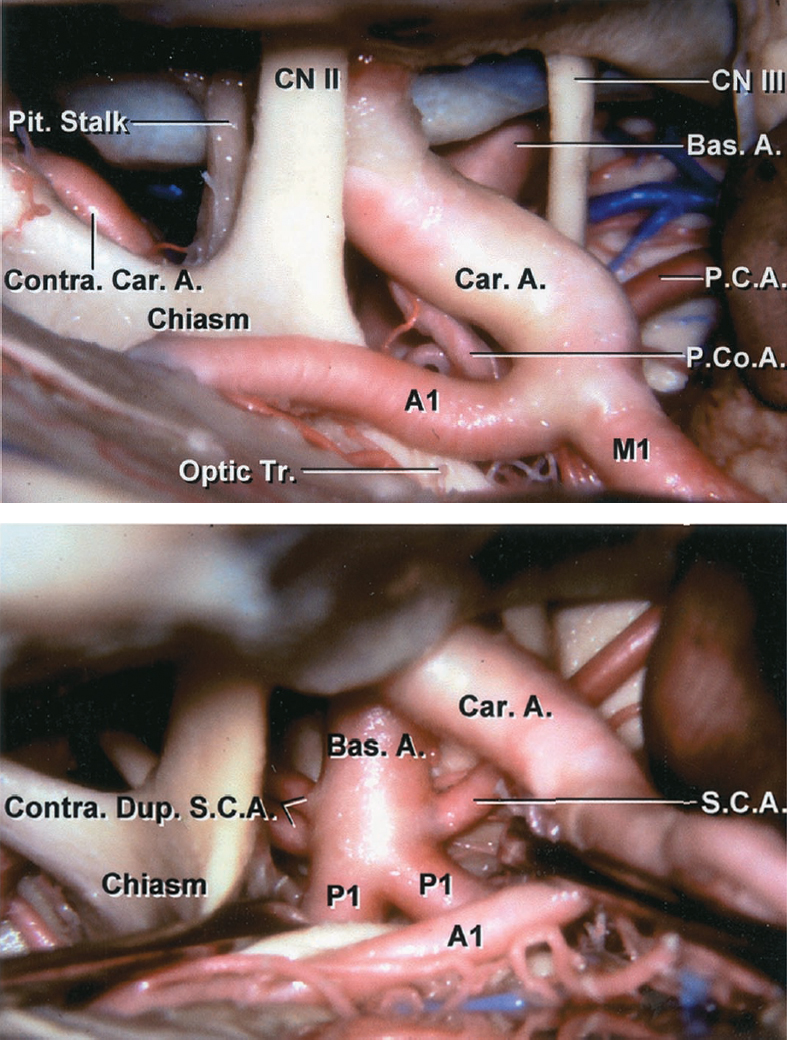
Figure 2: The right-sided operative pathway toward the basilar terminus region is shown. Note the working space through the opticocarotid triangle. The vascular anatomy of the basilar apex region is marked (images courtesy of AL Rhoton, Jr.)
Click here to view the interactive module and related content for this image.
Figure 3: The other two common operative corridors toward the basilar apex region are summarized. The supracarotid (upper images) and carotid-oculomotor pathways (middle row) are depicted. The subtemporal route is an alternative option (lower images); however it provides limited working angles and operative space (images courtesy of AL Rhoton, Jr.)
Figure 4: The opticocarotid and supracarotid pathways toward the basilar terminus territory are compared (upper images.) The A1 and M1 perforating vessels often render the supracarotid pathway unsafe. The subtemporal route can be extended via transection of the tentorium for low-lying basilar bifurcation aneurysms (lower images) (images courtesy of AL Rhoton, Jr.)
The posterior thalamoperforators arise from the P1 segment of the PCA and basilar bifurcation. Care should be taken to prevent injury to these vessels during dissection because they lie in close proximity of the aneurysm neck and supply the posterior thalamus, hypothalamus, reticular formation, and posterior internal capsule. In addition, there are circumflex perforators that arise from the P2 segment to course medially toward the brainstem. Similarly, thalamogeniculate and peduncular perforators arising from the P2 segment of the PCA should be protected. Life runs through these vital perforators that supply the mesencephalon and posterior diencephalon.
The Liliequist’s membrane is an important landmark during dissection of the interpeduncular cisterns. As the surgeon dissects around the posterior aspect of the communicating artery and P1 segment, this thick arachnoid sheet must be generously split just medial to the oculomotor nerve and in a stellate fashion for exposure of the basilar apex. The Liliequist’s membrane is stretched between the mammillary bodies and the dorsum sellae and laterally bordered by the oculomotor nerves.
MICROSURGICAL CLIP LIGATION OF BASILAR BIFURCATION ANEURYSMS VIA THE PTERIONAL ROUTE
Aneurysms arising from the basilar bifurcation may be approached using the extended pterional, modified orbitozygomatic or subtemporal approaches. Less commonly considered alternatives for approaching basilar trunk aneurysms include the combined supra- and infratentorial presigmoid transpetrosal osteotomy.
Special attention must be paid to the cranial-caudal location of a basilar artery aneurysm in relation to the dorsum sellae. Lesions within a 5-mm vertical span of the dorsum sellae are typically candidates for the pterional or modified orbitozygomatic routes. “High-riding” basilar bifurcation aneurysms (when the bifurcation occurs greater than 1 cm higher than the dorsum sellae) can be approached via a modified orbitozygomatic craniotomy.
As the pathology descends, it becomes necessary to modify the approach to match the lesion’s location. A temporopolar transcavernous approach with resection of the posterior clinoid and drilling of the dorsum sellae may be required for lower lying aneurysms.
I strongly favor the pterional or half-and-half (see below) approaches as opposed to the subtemporal approach. The former routes provide more flexible working angles to control the basilar artery prior to aneurysm dissection, and importantly, improve access to the contralateral P1 and superior cerebellar arteries. Although visualization of the perforating vessels at the basilar apex may be slightly compromised by the more frontal operative trajectory, I am easily able to reorient the operative view toward the temporal vector as necessary.
Finally, one should not undermine the efficacy of vertebral or basilar artery deliberate proximal occlusion in the management of these difficult lesions. This mode of therapy should be reserved for the aneurysms not amenable to endovascular or microsurgical therapy.
If intraoperative findings prove primary clip ligation unsafe, and robust posterior communicating arteries are present, clip ligation of the basilar artery at the its perforator-free zone is a reasonable consideration. The resultant hemodynamic changes can decrease the risk of future subarachnoid hemorrhage or even lead to intrasaccular thrombosis.
Choosing the Side of Approach
Basilar aneurysms are generally approached from the nondominant right hemisphere. Most surgeons are right-handed and prefer microsurgical maneuvers and clip application using their dominant hand. Moreover, the nondominant hemisphere more readily tolerates the temporal and frontal retraction necessary for the exposure. I am a left-handed surgeon and prefer using a left-sided craniotomy and take all of the necessary measures to avoid retraction injuries.
The presence of a second aneurysm or a hematoma directs the approach to that side. A preexisting third nerve palsy or hemiparesis would also direct the surgery to the corresponding side. Finally, the anatomy of the aneurysm, the relative position of the P1 shoulders at the level of the aneurysm neck, and the suspected location of the involved perforators also play a role in dictating the side of the approach. I approach the aneurysm from the side of the higher P1 shoulder so the contralateral P1 and its perforating vessels are less likely to be compromised by the less visible distal ends of the clip blades.
Patient Positioning and Craniotomy
Ideal positioning can minimize brain retraction and optimize exposure. The patient’s head is rotated away from the operative side by approximately 20 to 30 degrees. More or less rotation will increase the amount of retraction required on either the frontal or temporal lobes and will mandate increased manipulation of the ipsilateral internal carotid artery (ICA) and optic nerve. The head is extended so that the maxillary eminence is the highest point on the head.
A generous subtemporal craniectomy is necessary to expose the temporal tip. Improved exposure in the area will permit increased lateral and superior exposure around the temporal lobe, improving visibility through the interpeduncular cisterns. The squamous temporal bone must be drilled flush with the middle fossa floor. This maneuver is imperative because lateral retraction of the temporal lobe is a key part of the dissection of the basilar apex for the “half-and-half” modification described below.
An extensive resection of the sphenoid wing is performed to the level of the superior orbital fissure, providing a more anterior and inferior vantage point for reaching superiorly and posteriorly into the interpeduncular fossa. An extradural clinoidectomy increases the opticocarotid space via improved mobilization of the carotid artery. This maneuver should be considered for “high-riding” aneurysms.
Figure 5: The roof of the orbit is flattened and the sphenoid wing is resected to the level of the superior orbital fissure. Note the need for a generous subtemporal craniectomy that is an important part of the “half-and-half” approach for access to the temporal tip and its mobilization (see below.)
At this point, the dura over the sphenoidal and opercular sections of the Sylvian fissure is exposed. Bone wax is used on the bony edges to achieve hemostasis and prevent cerebrospinal fluid leak through the aerated medial sphenoid ridge, which occurs in 10% of patients. The dura is opened in a curvilinear fashion.
INTRADURAL PROCEDURE
Initial Exposure
The Sylvian fissure is widely dissected along both its horizontal and sphenoidal portions. Please refer to the Techniques of Sylvian Fissure Split chapter for more details. It is imperative not to minimize the importance of this step of the operation. The posterior subfrontal transsylvian trajectory allows examination of the posterior interpeduncular cisterns.
With exposure of the proximal carotid artery, I start a posterior arachnoid incision, paralleling the course of the posterior communicating artery and extending to the ipsilateral medial uncus. Medially, the arachnoid attachments to the gyrus rectus are disconnected. The lamina terminalis can also be fenestrated early to obtain further relaxation, especially in ruptured cases.
Next, attention is focused on achieving optimal temporal lobe mobilization. To do so, the temporal lobe must be freed from its attachments to the frontal lobe and sphenoidal dura. The veins draining into the sphenoparietal sinus along the temporal tip are coagulated and cut.
Dissection Toward the Interpeduncular Fossa
The next step of this approach depends on the patient’s individual anatomy. There are three potential routes for exposure of the distal basilar artery. The anterior aspect of the ICA at the skull base typically courses close to the optic nerve; therefore, the best exposure to the interpeduncular cistern is secured lateral to the optic nerve and the carotid artery and medial to the oculomotor nerve along the inferior aspect of the posterior communicating artery (the carotid-oculomotor triangle.) If the ICA travels more horizontally, I opt to use the space lateral to the optic nerve and medial to the ICA (the opticoarotid triangle).
In both of these pathways, the surgeon depends on mobilization of the ICA to maximize the operative corridor. If ICA mobility is limited secondary to a large atheroma, a third option is to use the corridor superior to the ICA bifurcation (the supracarotid triangle.) The major drawback with this approach is the limited maneuverability between the A1 and M1 perforators.
Figure 6: The most common operative corridors toward the interpeduncular fossa are the opticocarotid and the carotid-oculomotor triangles (green arrows). I prefer the carotid-oculomotor trajectory as long as the anatomy is favorable and undue traction on the oculomotor nerve is not necessary. The nerve should be untethered along its length.
Whichever approach is used, the ultimate goal of the next step is dissection and isolation of the posterior communicating artery as it joins the PCA. The posterior communicating artery travels through the membrane of Liliequist that should be widely incised to prevent unintentional traction on the perforators within the ambient cisterns.
Figure 7: The thick and minimally transparent membrane of Liliequist is incised. In this retrocarotid exposure, the inferior aspect of the posterior communicating artery is dissected and its attachments to the envelopes of the Liliequist’s membrane are divided. The anterior thalamoperforating vessels arising from the superior aspect of this vessel are left undisturbed.
Although some colleagues have considered sharp dissection of the arachnoid layers encasing the leash of thalamoperforating arteries to be relatively benign, I recommend against any manipulation of these vessels since the small caliber of these arteries dramatically exposes them to the risk of arterial wall dissection injury.
Once the junction of the posterior communicating artery and PCA are found, the P1 is followed medially toward the basilar artery bifurcation where the aneurysm is found. Dynamic retraction on the ICA leads to its intermittent stenosis, which is readily tolerable with no adverse consequences; however, the use of fixed retractor blades on the ICA is prohibited. The operator must be aware of the need for intermittent periods of reperfusion by relieving the traction force on the ICA.
The contralateral P1 and superior cerebellar arteries are unveiled first, before further dissection is contemplated around the aneurysm neck. The oculomotor nerve is the landmark that readily defines the identity of the superior cerebellar and posterior cerebral arteries. If the aneurysm is ruptured, the dense hemorrhage within the interpeduncular cisterns must be carefully evacuated to allow the operator’s orientation to the vascular anatomy, especially in the case of anterior-projecting aneurysms.
Figure 8: Next, I focus on securing proximal control on a perforator-free zone of the basilar trunk. I prepare the trunk above or below the origin of the superior cerebellar artery. The interval between the superior cerebellar artery and the PCA branches may often be short and not perforator-free. Application of the clip is attempted but not completed, just to make sure this maneuver can be repeated without any difficulty later and the bulk of the clip is not obstructive.
If proximal control is not possible because of a low-lying bifurcation, the posterior clinoid can be resected via drilling to expose the more proximal section of the artery. For relevant technical details, please refer to the chapter on superior cerebellar artery aneurysm.
Aneurysm Dissection
The origins of bilateral PCAs must be clearly identified because their superior borders/shoulders serve as landmarks for passage of the clip blades. The superior surfaces of ipsilateral and contralateral P1s are carefully inspected for vital perforators. I routinely use temporary occlusion of the basilar trunk and deflation of the sac to thoroughly mobilize the neck anteriorly. This key maneuver allows generous visualization of the posterior neck and sharp dissection of the basilar terminus perforators.
Aggressive manipulation of the perforators is avoided since this may lead to dissection of their arterial walls and their occlusion. Only the aneurysm neck is freed so the blades will not jeopardize the perforators; there is no need to dissect the perforators along their entire length. The aneurysm is retracted rather than the perforators. Although the bifurcation usually does not carry any perforators, the posterior aspect of the distal trunk does; these vital small feeding vessels travel a relatively long distance to supply the brainstem and diencephalon and can therefore be attached to the aneurysm.
Figure 9: One advantages of the pterional approach over the subtemporal approach is that it gives the surgeon the ability to fully inspect the aneurysm neck for perforators, particularly the component intimate with the contralateral PCA, which is not easily viewable via the subtemporal approach.
Clip Application
Once proximal arterial control is secured and the P1 origins and adjacent perforating vessels are meticulously isolated from the aneurysm neck, the plan for clip application can be investigated.
A few key principles apply to clip application of basilar terminus aneurysms. Primarily, the entire span of the clip blades should be visualized to confirm that none of the perforators is caught in the blades. I prefer the shortest clip configurations to minimize the risk of inadvertently encompassing the perforators, particularly in the area of the contralateral P1 origin. However, longer clips may be used if the working space is limited and the clip appliers obstruct an adequate view during application of the shorter clips.
The proximal perforators on the first few millimeters of the contralateral P1 are at the highest risk of compromise because they are in the surgeon’s operative blind spot during clip application. Their presence within the blades must be clearly excluded via careful inspection. The anatomic relationship of the shoulder of the contralateral P1 to the neck of the aneurysm also determines the horizontal slope of the clip blades so that the neck is adequately excluded from the circulation. Therefore, the origin of the contralateral P1 plays an important role for different reasons.
With large aneurysms, the origin of the contralateral P1 may be apparent only during the final few millimeters of clip closure. Minor adjustments can be made to reorient the clip blades to avoid any compromise of this efferent vessel. Clip repositioning after its initial application is not problematic, but too many adjustments can weaken the integrity of the aneurysm neck and lead to a neck tear.
The projection of the dome has important implications for selection of the appropriate clip configurations. Anterior-projecting aneurysms are often managed via simple straight clips because the perforating vessels are readily dissectible from the neck. Fenestrated clips can encircle high-riding P1 origins and ipsilateral perforating arteries. Posterior-projecting aneurysms are excluded using tandem fenestrated clips; the first clip allows visualization of the shoulder of the contralateral P1. Superiorly-projecting aneurysms can be repaired using a combination of straight and fenestrated clips.
The superior dome projection hides the perforators at the distal neck, although the contralateral PCAs are visualized. The anterior dome projection hides the contralateral PCA. The posterior projection is the most challenging because the perforators are adherent to the dome and the neck is obscured.
Figure 10: My favorite clip configuration is a fenestrated clip loaded on a side-angled clip applier, encircling the ipsilateral P1. I often need to dissect the fissure more posteriorly to achieve an appropriate operative trajectory parallel to the posterior neck of the aneurysm. Temporary placement of a fixed retractor blade on the temporal lobe may be necessary to allow flexible working angles during final adjustment of the clip blades. I spend a reasonable amount of time adjusting the blades’ final location during slow closure of the clip.
The mouthswitch of the microscope provides the required shift in the field of view and line of sight while the clip is adjusted and keeps the image in focus so that the perforators remain visible. This functionality of the microscope is imperative so the operator can use both hands at all times, using the nondominant hand and the suction device to contour the neck into the clip blades and gently mobilize the perforators for safe and effective aneurysm obliteration. The line of sight may need to be adjusted from being directed toward the retrocarotid region to being focused on the opticocarotid triangle and vice versa.
Figure 11: Once the clip is deployed and inspection reveals no obvious evidence of perforator compromise, an intraoperative fluorescence angiogram is performed to confirm a lack of flow into the sac and preservation of the adjacent branching and perforating arteries. As the neck cross section becomes oval after the initial clip application, the surgeon may find the clip to be too short and clip exchange may be necessary. This is the most common cause for persistent filling of the sac. If the sac is not fluorescent, the dome is deflated using a needle.
Figure 12: Mobilization of the deflated sac provides the much needed viewing angles to convincingly confirm preservation of all the perforating vessels behind the neck. The location of the tips of the blades just over the shoulder of the contralateral P1 is also confirmed.
Figure 13: A basilar bifurcation aneurysm was exposed via the left-sided transsylvian approach (the Liliequist membrane and the origin of the PCoA (arrow) are shown)(first row). The PCoA was pursued through the Liliequist membrane and the oculomotor nerve released from its arachnoids bands (second row). The leash of PCoA thalamoperforating vessels was gently mobilized and proximal control over the basilar artery secured (third row). The temporal lobe was gently retracted momentarily using fixed retraction while a straight permanent clip was deployed across the neck (fourth row). Careful inspection revealed that the contralateral P1 origin and its associated perforators were safe. The use of dynamic retraction minimized any cortical injury (lower row).
Figure 14: The tips of the blades should not promise the contralateral P1 proximal perforators (arrow). This basilar aneurysm was approached through a right-sided transsylvian route.
Other Considerations
If the posterior clinoid process obstructs the view of the neck or the ability to secure proximal control, a partial posterior clinoidectomy can be performed. With the dura of the posterior clinoid reflected, the clinoid is cored out and its remaining thin shell removed. Venous bleeding from the cavernous sinus is managed using packing of thrombin-soaked Gelfoam powder. This procedure is not without risks, and I have heard of numerous events when vital deep perforating vessels were inadvertently sacrificed by the drill’s rotational force.
Given the redundancy in the circle of Willis, it may be possible to disconnect the P1 or posterior communicating artery based on their caliber relative to each other. The communicating artery may be shapely disconnected at its attachment site to the ICA or P1 using small metal clips. Either of these maneuvers might improve visibility of the aneurysm neck based on the anatomy of the surrounding region. I have not used this maneuver and advise against its use because of the potential risks to the perforating vessels that are involved.
I do not feel ashamed to abort the microsurgical procedure and resort to endovascular treatment if the anatomy is deemed unfavorable upon intraoperative inspection. The following intraoperative factors will lead me to abort the procedure to protect the patient from unnecessary risks: an aneurysm arising from a high basilar artery bifurcation; cartilaginous hypertrophy of the clinoid process (invisible on the preoperative CT); an immovable, large atheromatous carotid artery; or a very posterior-projecting aneurysm that renders inspection of the perforators unsafe.
Some colleagues may consider proximal occlusion of the basilar artery in the presence of competent posterior communicating arteries. This strategy can be effective if the resultant alterations in the hemodynamics of basilar bifurcation lead to delayed sac thrombosis.
The “Half-and-Half” Approach
Finally, the subfrontal approach can be converted to a “half-and-half” approach in which the direction of the temporal retractor blade is changed from retracting in the posterior direction to retracting in the posterior-superior direction.
A second, slightly wider, temporal retractor blade is placed on the temporal tip, lifting the tip laterally and superiorly, providing excellent visibility of the oculomotor nerve and tentorium posteriorly around the interpeduncular cistern. The oculomotor nerve is untethered from the medial temporal lobe. The microscope’s line of sight is then inclined to provide an inferior viewing trajectory toward the clivus. High bifurcation and posterior-pointing aneurysms are readily exposed through the half-and-half approach, obviating the need for the other options discussed above.
Variations
The anatomy of the P1 arteries in relation to the aneurysm neck and the available working angles demand very flexible clip application strategies for obliteration of the aneurysm while preserving the adjacent perforating arteries.
Figure 15: Tandem overstacked clipping of a basilar artery bifurcation aneurysm is evident. The proximal tentative fenestrated clip is placed first, occluding the distal portion of the aneurysm neck and improving my visualization of the aneurysmal anatomy. The second, straight clip is placed just distal to the fenestrated one, occluding the remaining portion of the aneurysm neck and contouring the reconstruction of the neck while giving me more control to maneuver the smaller clip to preserve the perforating arteries.
Figure 16: Tandem understacked clipping of a basilar artery bifurcation aneurysm is very beneficial. The fenestrated clip is placed distally on the aneurysm, closing off the most distal portion of the neck. The second, simple clip is deployed just proximal to the fenestrated clip, collapsing the remaining portion of the neck. The efferent arteries are readily spared while appropriate neck exclusion is rendered.
Figure 17: The tandem overstacking technique for ligation of a basilar artery bifurcation aneurysm with thick walls is illustrated. An additional booster clip is also applied. The proximal fenestrated clip was placed first, occluding the distal portion of the aneurysm neck and improving visualization of the aneurysmal anatomy. The second, simple clip was then placed just distal to the fenestrated one, occluding the remaining portion of the neck. Intraoperative fluorescence angiography demonstrated persistent filling of the aneurysm. Next, a booster clip was positioned just distal to the straight clip, ensuring that the aneurysm is completely closed off.
Figure 18: A basilar artery aneurysm is clipped with a tandem overstacking method to allow for retrograde flow into the efferent P1 segment of the PCA. The blood flow is indicated with the green arrows. The aneurysm is first clipped using a fenestrated clip on its proximal neck. A second fenestrated clip is placed just distal to the first one, creating a lumen for blood flow. Finally, a simple clip is placed just distal the second fenestrated clip, rerouting the flow into the efferent P1 segment rather than the dome.
Figure 19: Tandem overstacked clipping of a basilar artery bifurcation aneurysm (first row) is shown. The posterior clinoid was drilled (second row). Proximal control was established and the perforator at the distal neck was dissected (third row). The perforators on the posterior aneurysm wall were recognized and a straight fenestrated clip was applied, leaving the ipsilateral P1 and proximal perforating vessels in the fenestration (fourth row). The initial clip blades spared the origin of the contralateral P1 and an additional straight clip closed the remaining proximal neck (final row).
Pearls and Pitfalls
- The anatomy of the interpeduncular cisterns is complex and should not be taken for granted. More than one anatomic landmark should be used for reliable intraoperative orientation.
- I recommend dissection of almost all basilar apex aneurysms under proximal temporary occlusion.
- Each perforating vessel of the basilar apex territory carries life through it. Meticulous dissection of the neck and perforator preservation cannot be overemphasized.
Contributor: Christopher Kellner, MD
References
Batjer HH. Aneurysms of distal basilar artery, the pterional approach, in Samson DM (ed): Intracranial Aneurysm Surgery: Techniques. Mount Kisco, NY: Future Publishing, 1990.
Lawton MT. Basilar artery bifurcation aneurysms, in Seven Aneurysms: Tenets and Techniques for Clipping. Thieme, 2011.
Please login to post a comment.