Low-Grade Glioma
Figure 1: Walter Dandy demonstrated the techniques for resection of intraparenchymal tumors (Dandy WE. The Brain. Hagerstown, MD: WF Prior Company, 1966).
Please note the relevant information for patients with gliomas is presented in another chapter. Please click here for patient-related content.
This is a preview. Check to see if you have access to the full video. Check access
Frontal Glioma: Basic Dissection Principles
World Health Organization (WHO) grade I & II tumors of glial origin are considered low-grade gliomas (LGGs). There are three common types of LGGs, namley astrocytomas, oligodendrogliomas, and oligoastrocytomas. These tumors originate from one or both of the two glial cell types: astrocytes or oligodendrocytes. The extent of resection is favored to be one of the most influential determinants of overall survival, progression-free survival, and malignant transformation. However the selection bias reflected in the corresponding studies demands judicious application of the published data to current practice.
These tumors are typically discovered in young patients (20-64 years of age, with a median age of 39) who are otherwise without comorbidities and with many expected productive years of life, at the time of diagnosis. Although LGGs are more radiographically and histologically less aggressive than their high-grade counterparts, many patients still eventually die of their disease because of tumor progression and malignant transformation. Hence, safe maximal tumor resection can provide patients with the best opportunity for excellent quality and length of life.
Diagnosis
Approximately 60–80% of patients with LGGs present with seizures of generalized semiology. Focal seizures, headaches, or progressive neurologic symptoms such as weakness, sensory loss, apraxia, or aphasia can also be among the presenting symptoms and signs
These tumors are rarely diagnosed incidentally on imaging performed for trauma or other unrelated reasons.
Evaluation
Usually a computed tomography (CT) scan or magnetic resonance imaging (MRI) is the first study to reveal the tumor; this depends on the location of first evaluation (emergency department, inpatient hospitalization versus outpatient visit). An MRI with and without gadolinium contrast is required for fine-detail distinction and assessment of the extent of the tumor and its contrast uptake. Remarkable enhancement is consistent with higher grade tumors.
LGGs tend to be non-enhancing (especially grade II tumors). However, pilocytic astrocytoma, an astrocytic LGG, is a cystic tumor with an enhancing mural nodule. Optic apparatus gliomas are diffusively infiltrating LGGs causing enlargement of optic nerve, chiasm or tract, with variable patterns of enhancement. Vasogenic edema is not a prominent feature of these lesions, in stark contrast to high-grade gliomas.
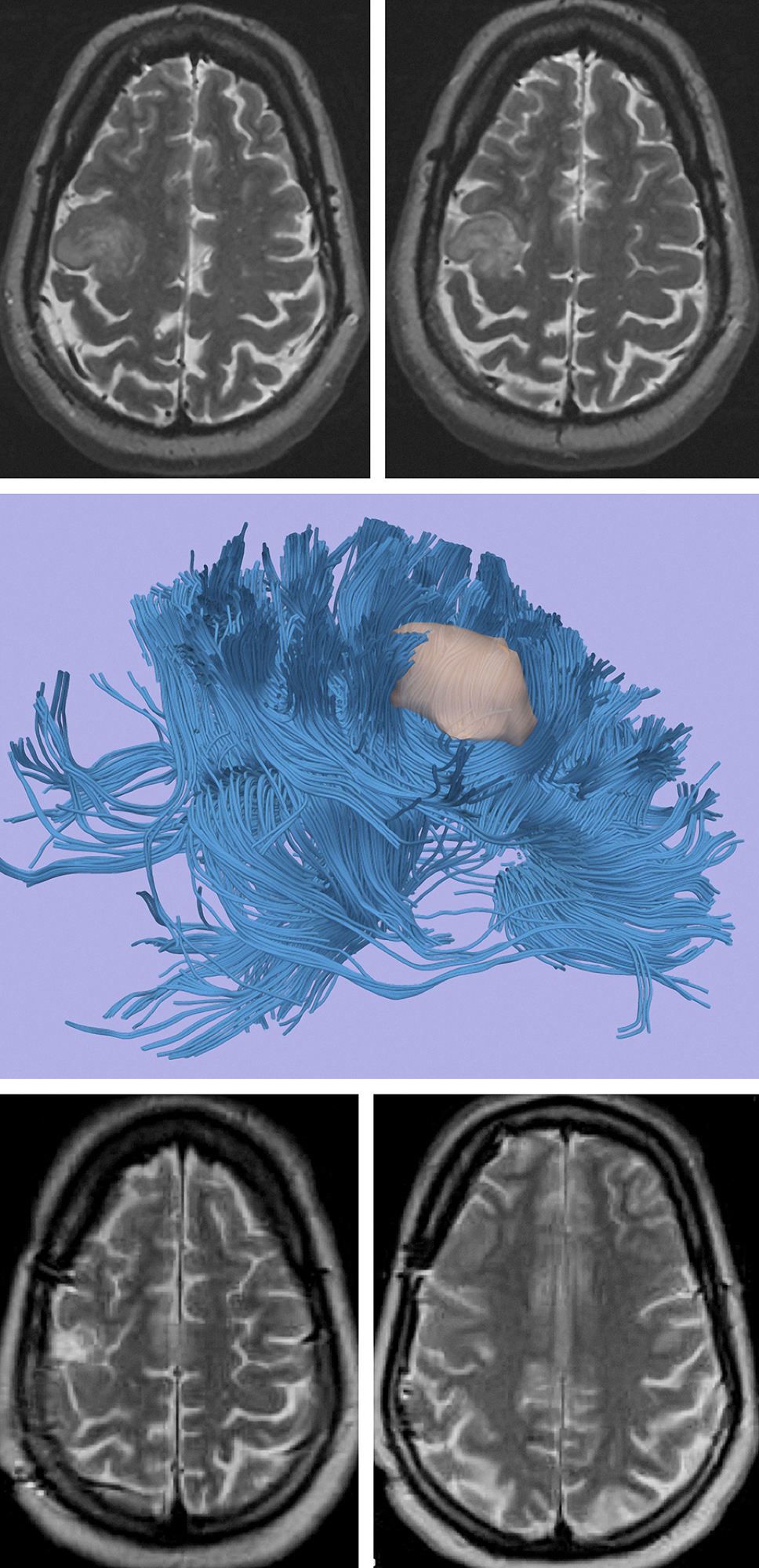
Functional MRI is important for operative planning is select patients. This modality provides valuable information about the relationship between the adjacent eloquent tissue and the lesion; however, it does not replace intraoperative brain stimulation mapping for tumors closely involved with vital cortices. Other studies, such as diffusion tensor imaging (DTI), map the white matter tracts around the tumor and estimate the degree of their infiltration.
Figure 2: Classic findings for a nonenhancing well-defined cortically-based LGG. Evidence of punctate calcification and a somewhat heterogeneous appearance on these T2 sequences are consistent with a diagnosis of an oligodendroglioma.
Large Dominant Frontal Glioma: Awake Mapping Techniques
Figure 3: A LGG located in close proximity to the motor cortex (upper images). Functional MRI and DTI revealed close proximity of the lesion, but its distinct borders relative to the vital tracts (middle image). Intraoperative mapping guided gross total resection of the lesion without any new neurological deficits (lower images).
Indications for the Procedure
The indications for resection (versus biopsy alone) include reliable tissue diagnosis without the risk of sampling error, control of medically refractory seizures, prevention of malignant transformation through cytoreductive surgery, and improvement in the patient’s survival.
Resection of large well-delineated superficial lesions within silent regions or frontotemporal poles of the brain is universally indicated, especially in younger patients. Resection of tumors in the vicinity of eloquent areas is recommended, but must be individualized based on the location and diffuseness of the tumor.
Some operators believe that at least >80% of the tumor should be potentially resectable in order to justify the risk-to-benefit ratio for considering microsurgical excision. Diffuseness and the location of the tumor dramatically affect the role of surgery. Multifocal tumors are more likely to be biopsy candidates.
Preoperative Considerations
Low-grade glial tumors are often not associated with surrounding edema. Pre-, intra-, and postoperative use of steroids are controversial, but often administered; perioperative anticonvulsant medications are strongly recommended.
The decision to perform an awake versus sleep mapping, or standard craniotomy must be considered, influenced by the eloquence of the involved cortices. Please refer to the sections on Language Mapping for Glioma and Sensorimotor Mapping for Glioma for important details regarding preparation, craniotomy, and other operative considerations.
RESECTION OF LOW-GRADE GLIOMA
A reasonable number of patients will require a subsequent reoperation for recurrent tumors. The initial incision should therefore be carefully planned. Linear incisions offer the most flexibility and robust vascular support for future reoperations.
Although small linear incisions are desirable, some patients are better served by a larger incision designed for aggressive tumor resection while accessing both the lesion and the surrounding normal cortex for mapping and electrocorticography.
For general details regarding exposure and craniotomy, please refer to other sections of this Atlas, including the Cranial Approaches volume. Intraoperative Navigation guides the extents of an adequate craniotomy.
INTRADURAL PROCEDURE
Low-grade glial tumors are minimally vascular and relatively simple to excise.
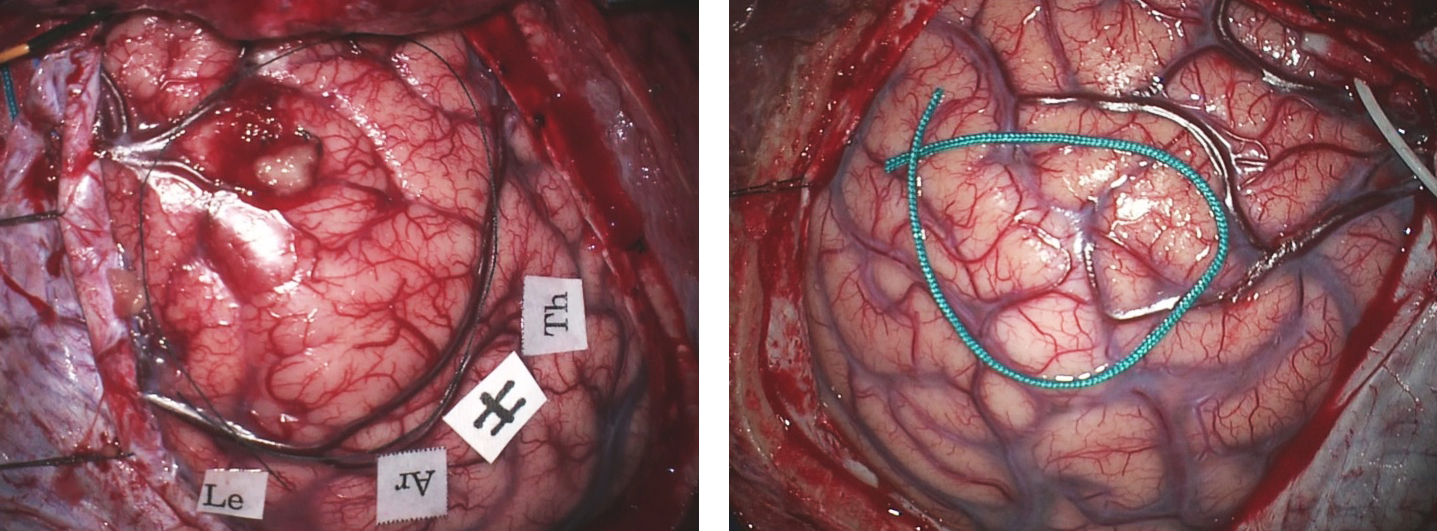
Figure 4: The dura may be opened in either a curvilinear or stellate fashion. The exposure must reveal a generous surface of the brain involved with the tumor and the surrounding normal cortices. A ribbon of relatively normal-appearing cortex often covers the edges of the tumor.
Before brain shift (caused by tumor debulking and/or release of cerebrospinal fluid) occurs, I use navigation data and mark the tumors’ boundaries using a piece of silk suture. A detailed inspection of the brain surface reveals that the involved gyri are expanded, discolored and appear hypovascular. If necessary, cortical stimulation mapping can precisely localize the boundaries of functional cortices. The surrounding large veins and arteries are identified and preserved.
Figure 5: Cortical LGGs may (left) or may not (right) cause changes in their overlying cortical morphology. In the left image, the right frontal premotor cortices are expanded and the parasagittal veins are displaced posteriorly. The right posterior frontal tumor in the right image (bounded by the green suture) is causing minimal alteration in the cortical morphology.
Figure 6: Initial subpial coagulation marks the superficial tumor edges and devascularizes the surface of the tumor. En passage cortical arteries and veins are protected. A piece of tumor is sent for histologic examination and confirmation of the preoperative diagnosis.
Some LGGs have diffuse edges and are difficult to fully delineate from the surrounding normal/functional tissue, whereas others are more definitively demarcated. In other words, some LGGs are gray, soft, suctionable, and have distinct textures; these features significantly facilitate their gross total resection. Many other tumors are relatively fibrous and have heterogeneous consistency that complicates their resection because the operator has no reliable method to differentiate the tumor from the peritumoral tissue.
Gentle dynamic maneuvers to mobilize the surrounding brain minimize retraction injury. Subpial resection of the tumor along the medial interhemispheric or Sylvian fissure opercula is conducted.
Figure 7: I prefer to circumferentially disconnect the tumor as guided by the tumors’ consistency and intraoperative navigation. I also attempt to remove the tumor en bloc, if possible. Central debulking leads to a greater loss of navigational accuracy. Importantly, en bloc removal of the tumor advances technical efficiency, minimizes blood loss, and allows the operator to keep his or her orientation while maintaining the resection planes along the tumor margins. Working simultaneously on both the inside and the periphery of the tumor can lead to confusion about the tumors’ pseudo margins.
The suction apparatus can be used as a vector of dynamic retraction to prevent the wall of the resection cavity from collapsing.
The technique of white matter dissection and disconnection of glial tumors is worth special emphasis. The bipolar forceps repeatedly grab and coagulate the pseudo capsule. This stepwise maneuver leads to emulsification of the pseudo capsule and its disconnection from the peritumoral edematous tissue. Next, the suction apparatus removes this emulsified material and exposes the next layer of the pseudocapsule for further coagulation and disconnection.
The above technique disconnects and coagulates simultaneously. In other words, the bipolar forceps effectively act as tumor scissors via the repeat spring action of the forceps’ tips while coagulating (above image, figure 7, inset).
The response of the tumor to bipolar coagulation can be quite different than that of the normal brain; this is another important parameter that can guide the surgeon. Some LGGs are more fibrous and some are even more gelatinous that normal brain. The distinction along the peritumoral region can be quite challenging.
Figure 8: The tumor is finally disconnected at the depth of the cavity and removed. Navigation is then used to estimate the extent of resection. Further resection within the walls of the cavity is necessary to achieve reasonable goals. This maneuver is continued until clean white matter (bright and glistening) is encountered in all directions. The tumor often appears dull. LGGS often extend to the level of the ependyma and entry into the ventricle confirms removal of the deepest portion of the tumor.
Circumferential Sulcus-Guided resection Technique
Because low-grade gliomas do not migrate through sulci, the sulci help demarcate the tumor edges, and the circumferential sulcus-guided resection technique leverages this normal tumor growth pattern to the advantage of the surgeon. Circumferential sulcus-guided resection of low-grade gliomas is safe and feasible, even in eloquent locations, and is independently associated with a higher rate of complete resection than is piecemeal resection.
These are the involved steps for circumferential sulcus-guided resection:
Step 1: Spatial assessment to define the tumor boundaries
Step 2: Functional assessment to localize eloquent brain
Step 3: Sulcus-guided anatomical circumferential resection
Step 4: White natter dissection
Figure 9: Cross-section depiction of the brain showing how tumor can expand a gyrus and bulge underneath a neighboring gyrus. Although the neighboring gyrus appears to involve tumor when viewed from the surface, in fact the tumor edge is defined by the sulcus and tumor does not invade into the neighboring sulcus. When this expansion occurs near a positive stimulation site, it may appear that the tumor under the site cannot be resected, but in fact it can be. The boundaries of dissection are outlined in green.
Figure 10: The circumferential sulcus-guided resection technique is based on the observation that low grade gliomas in general do not cross sulci, and when they invade a neighboring gyrus they are likely to do so by following the short U-fibers. As a result, the boundaries of low grade gliomas can be defined largely by the sulci that surround the hyperintense tumor as seen on FLAIR/T2-weighted MRI. The gold plane refers to Fig 9 above.
Sulcus-guided anatomical circumferential dissection begins by opening the arachnoid over the sulci and splitting the surrounding sulci. This opening is usually done with sharp dissection or by gentle spring sction of bipolar forceps. Overlying veins are preserved if needed.
Figure 11: Specifically, resection proceeds by dissecting down the sulci along the pia or by opening the sulci that surround the tumor. Once at the base of the sulcus, the interface between tumor and white matter is identified, and the bottom of the tumor is dissected. Importantly, the large vessels within the sulci are preserved and the small perforating vessels that enter the gyri/tumor are coagulated and cut, resulting in progressive devascularization of the tumor.
Figure 12: Once the base of the sulci are reached, the tumor is dissected along the white matter by using navigation guidance to help define the tumor edge. Direct subcortical stimulation can be used to avoid injury to the descending motor fibers. As noted in this illustration, the boundaries of gliomas (infiltrative) at the level of the white matter are much less distinct. Dissection at the level of the white matter requires careful observation of textural and colors changes as well as surgical judgement, augmented by subcortical mapping and image-guided navigation.
Other Intraoperative Considerations
Many LGGs respect the pial surfaces of their gyri, but not the underlying white matter tracts. The accuracy of navigation drops precipitously at the deeper portions of resection cavity due to the brain shift caused by tumor debulking. The surgeon’s experience in using the tumor’s texture and response to suction in guiding resection can become an important element to ultimately determine the extent of removal on postoperative imaging.
Unfortunately, there is no reliable and practical method to guide tumor removal for LGGs along their edges; these edges frequently appear and feel almost the same as normal brain or tend to infiltrate normal functional cortices and white matter tracts. The surgeon should become very familiar with the tumor’s texture during the early parts of the operation and use this information for handling the final more indistinct periphery of the tumor. The ventricle is often a good landmark to define the deeper margins of the resection cavity.
Intraoperative MRI (iMRI) may be helpful, but in my opinion, is an inefficient use of operative time and cannot replace the surgeon’s experience.
Some tumors engulf vessels (such as the middle cerebral artery complex). Aggressive use of the suction tip in these regions may lead to vascular injury to the small perforating vessels and should be avoided. The operator’s enthusiasm for aggressive tumor removal must be balanced against the risk of injury to these vital vessels. Currently, there is no surgical method that can claim complete removal of LGGs. Therefore, preservation of function is the first priority in managing these tumors.
Closure
The principles of closure are the same as those discussed in the Cranial Approaches volume.
Postoperative Considerations
A postoperative MRI is obtained within 48 hours of resection to assess the extent of tumor removal. A critical, honest, and meticulous review of this study is necessary for advancing the surgeon’s learning curve for improving tumor removal for the future patients.
Steroids are weaned slowly as tolerated. Anticonvulsants are administered prophylactically (or increased in dosage) during the postoperative period.
Pearls and Pitfalls
- The operator’s experience in using the texture and color of the tumor to ensure maximal resection cannot be underestimated. The response of the tumor to bipolar coagulation and suction compared with the response of normal brain tissue is another important parameter that can guide the surgeon.
Contributor: Gina Monaco, MD
Please login to post a comment.