Language Mapping for Glioma
Figure 1: Cushing’s own illustrations show his passion for cortical electrophysiology. Forester, Penfield, and Boldrey further refined the techniques of cortical stimulation and shaped our understanding of this method. There is some controversy if Cushing had intended to sketch the face of William Osler in the left image.
This is a preview. Check to see if you have access to the full video. Check access
Large Dominant Frontal Glioma: Awake Mapping Techniques
The goal of glioma surgery is to maximize tumor resection while avoiding neurologic morbidity. Other advantages of resection include a decreased risk of sampling error associated with the biopsy procedure, seizure control, improvement of symptoms caused by mass effect, and in the case of low-grade gliomas, a decreased risk of future malignant transformation through cytoreductive surgery. The extent of resection correlates with time to progression and overall survival.
The greatest risk of tumor recurrence occurs within 2 cm of the tumor margin or contrast-enhanced region seen on magnetic resonance imaging (MRI). Therefore, one may conclude that the ideal resection should extend slightly beyond the tumor borders based on imaging, if deemed safe. The limiting factor is the potential loss of survival advantage if a postoperative neurologic deficit is inflicted. Clearly, a safe surgery while removing the maximal amount of tumor is the goal.
The vicinity of functional cortices and white matter tracts often limits the extent of resection within the adjacent tumor-infiltrated brain. To circumnavigate eloquent cortex and white matter, cortical stimulation is used to precisely localize or map function. During this form of stimulation, a very focal region of the cortex is depolarized, resulting in certain physical effects leading to transient localized cortical dysfunction. Specifically for language function, speech arrest when naming is used as an indicator of Wernicke’s area because naming difficulty is a major component of most aphasias.
Indications for the Procedure
The “awake” craniotomy under conscious sedation is typically advocated for resection of low-grade gliomas (LGGs) and less frequently for high-grade gliomas (HGGs) involving and/or adjacent to the eloquent regions of the brain. Occasionally, cerebral metastases in eloquent areas may benefit from cortical mapping so that the corticotomy and operative trajectory are planned safely, so that the surgeon avoids the functional gyri and tracts.
As a general rule, gliomas involving the dominant mid to posterior temporal, mid to posterior inferior frontal, posterior frontal and anterior parietal lobes should be considered for an awake mapping procedure. In other words, infiltrative tumors associated with the Broca’s and Wernicke’s cortices, as well as rolandic cortex, supplementary motor area, corona radiata, internal capsule, and uncinate fasciculus are suited for mapping.
Vascular lesions rarely require an awake method. AVM operations can be complicated by excessive blood loss are often lengthy, demand very rigid head immobilization, and require general anesthesia to control pCO2, brain oxygen metabolism, and blood pressure; these parameters cannot be reliably accomplished without intubation.
I use awake mapping primarily for LGGs. It has been my experience that resection of HGGs infiltrating the functional cortices and tracts often leads to neurologic morbidity despite preservation of these functional tissues. Subtotal resection of HGGs is associated with postoperative decline and risk of hematoma formation. However, I do use mapping for specific HGGs that do not directly infiltrate the functional areas, but are adjacent to these areas. This algorithm allows mapping to secure resection of immediate nonfunctional peritumoral borders in expectation of radical tumor removal.
I use awake mapping for both dominant and nondominant insular tumors and those intimately associated with the central lobule (sensorimotor cortices). Tumors that are nearby but do not directly infiltrate the central lobule can undergo sleep mapping. Awake mapping provides the most reliable and precise mapping method with minimal interference from anesthesia. Sensorimotor Mapping for Glioma and its indications are discussed in another chapter.
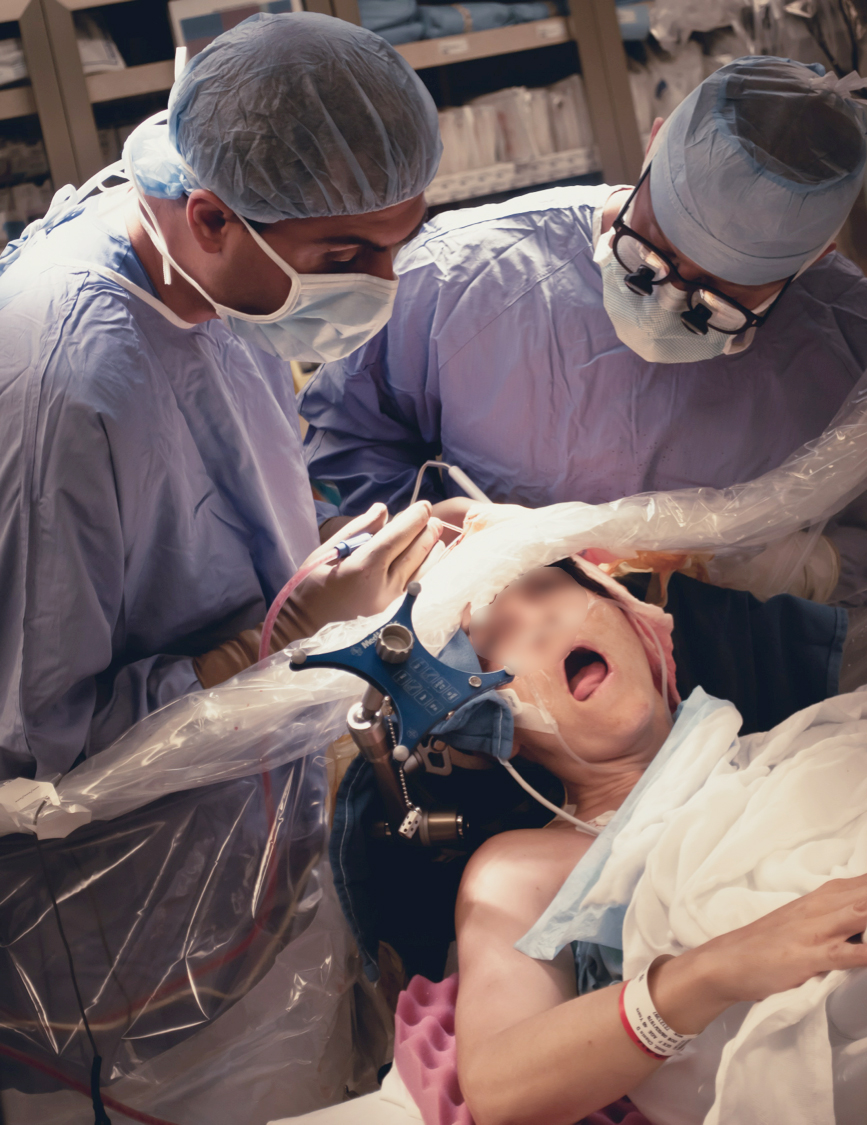
Figure 2: Mapping the face area. The patient must be comfortable. The patient-surgeon interaction is the key to success.
Appropriate patient selection is critical for successful execution of an awake craniotomy. The patient must be cooperative for 4-5 hours with a calm demeanor. Anxious, uncooperative, cognitively-compromised patients, or those with significant baseline language or motor deficits will fail awake procedures. Preoperative language function is evaluated for a naming failure rate of <25% using slides that start with ‘this is a …’ or ‘these are…’ to concurrently test reading functions. Each slide is tested three times.
The motor aspect of speech is evaluated by asking the patient to count from 1-10 and then to protrude the tongue. If the patient is suffering from significant deficits, he or she may be retested a week later after treatment with high-dose steroids or be operated on asleep with a goal of tumor debulking only.
Preoperative Considerations
Broca’s and Wernicke’s areas can be anatomically estimated on preoperative imaging to assess their involvement with the tumor and the need for an awake surgery; fMRI can assist with this task. Deep tumors may require mapping of the overlying cortex for selection of an appropriate operative trajectory.
Figure 3: The deep and posteroinferior frontal location of this tumor required awake speech mapping to guide a safe transcerebral corridor to reach the tumor (upper images). Development of a neurologic deficit can blunt or eliminate any survival advantage gained by resection of this high-grade glioma. Following a left-sided craniotomy, the most inferior location of motor speech was mapped (Lex)(middle image) and a corticotomy, just anterior to this eloquent cortex, was made to reach the tumor. Ultimately, gross total resection of the tumor was performed safely (lower images).
Functional imaging studies including fMRI are increasingly popular as an alternative method to map function. These imaging modalities do not replace the gold standard technique of cortical stimulation mapping. Instead, they guide the surgeon to map function more efficiently intraoperatively, rather than conducting an exploratory mapping strategy. I do not recommend resective surgery based on only fMRI data.
In addition, although fMRI may be reliable for identifying sensorimotor cortices and may obviate the need for cortical stimulation in a small number of patients, it is much less reliable for identifying language functions. In fact, the sensitivity for positron emission tomography (PET) and fMRI for correctly identifying the location of function are ~75% and 81%, respectively, and their specificities are 81% and 53%, respectively.
Most people are left hemisphere dominant, including those who are left handed. Among right-handed people, 98-99% are left hemisphere dominant. Only around 19% of left-handers have right-hemisphere language dominance, and another ~20% process language functions in both hemispheres. Moreover, the incidence of language distribution among ambidextrous people is broadly similar to that found in left-handed individuals.
Overall, ~93% of all people demonstrate left hemisphere language dominance. If there is any question regarding the cerebral dominance in a left-handed patient, a Wada test or fMRI should be performed.
Preoperative assessment also included language assessment by the surgical neurophysiology team that will be assisting with stimulation mapping during the surgery. The patient is counseled about the process of an awake surgery and mapping ahead of time. Intraoperative testing employed images and words that the patient was able to name or read during preoperative evaluation.
Operative Anatomy
An understanding of cortical functional anatomy is beneficial in estimating the location of function with respect to the tumor.
Click here to view the interactive module and related content for this image.
Figure 4: Left hemisphere and cortical anatomy related to language and sensorimotor function are shown (upper image). The classic cortical sites for language function are marked. Broca’s area lies in the posterior aspect of the inferior frontal gyrus. Wernicke’s area includes the perisylvian region in the left temporoparietal cortices. Note that there is a gyral bridge (red arrow) between the precentral and postcentral gyri so the sensorimotor cortices do not directly open into the Sylvian fissure (Upper image courtesy of AL Rhoton, Jr).
The M-sign (lower image) signifies Broca’s area and should be identified on preoperative imaging to determine its involvement with the tumor and the need for an awake surgery. The “M” can be identified on sagittal sequences as an M-shaped gyrus formed by the pars orbitalis, triangularis, and opercularis (lower image). This region contains the motor or expressive component of speech in 87-89% of patients.
The locations of the cortical language centers (comprehension or receptive speech) are incredibly variable. In fact, the resections guided by purely standard anatomic landmarks (ie anterior 4 cm of the dominant temporal lobe) have led to postoperative language deficits.
Congenital and long-standing benign lesions such as AVMs, cortical dyplasias and some LGGs can alter or transfer the distribution of cortical function. This phenomenon provides an excellent opportunity for aggressive excision of tumors and lesions otherwise deemed inoperable.
AWAKE MAPPING FOR RESECTION OF GLIOMAS ASSOCIATED WITH SPEECH CORTICES
Neuroanesthesia
Suitable neuroanesthesia aimed at keeping the patient comfortable is critical for success of an awake craniotomy. Two methods may be used to accomplish anesthesia for awake procedures. Some surgeons prefer the patient to be relatively awake during the entire procedure. Others prefer that the patient be awake only for the mapping and resection stages. Deeper levels of conscious sedation can lead to patients’ confusion and compromise his or her cooperation. I prefer to keep the patient heavily consciously sedated during exposure (craniotomy) and closure but only minimally sedated during the mapping stages.
The patient must be kept calm, but awake. Premedication may decrease the reliability of language testing during the procedure, but anxiety, hypertension, and Valsalva events may increase cerebral tension and herniation through the craniotomy defect.
A fine line must be straddled to maintain the appropriate level of sedation for several hours. Typically, premedication is accomplished through midazolam (Versed) and propofol (Diprivan) as well as fentanyl to maintain a sedated hypnotic state. During skull clamp placement, remifentanil (Ultiva) boluses may be used after injection of a generous amount of local anesthetic at the pin sites. After mapping is completed, sedation with dexmedetomidine (Precedex) and remifentanil is maintained until the conclusion of surgery. Sedation is reached using propofol (up to100 mcg/kg/min) and remifentanil (0.07–2.0 mcg/kg/min). Patients who do not tolerate propofol (causing apnea) may be given dexmedetomidine (loading dose of 1 mcg/kg IV over 10 minutes and maintenance dose of 0.6 mcg/kg/hr IV titrating to effect (usually 0.2-1.4 mcg/kg/hr).
A Foley catheter is inserted and mannitol is administered.
Patient Positioning and Craniotomy
Patient comfort is paramount to maintain control over the flow of the operation. There must be a compromise between the position required for the surgeon to safely conduct the operation and the position that delivers the most comfort to the patient. Ultimately, the latter is more important since it determines the patient’s tolerance for the procedure.
The head rotation and extension must be adjusted to avoid neck discomfort while allowing the patient to swallow comfortably. If the patient has a history of back pain, pillows must be placed under the knees and/or the back. All pressure points must be very well padded.
A warming blanket may be used to minimize shivering and movement of the operative target during microsurgery. Video monitors displaying the operative field are turned away from the patient’s line of sight.
Although most patients are placed in a supine position, there are rare circumstances when I use a lateral position to avoid significant patient’s neck torsion. Lesions in the posterior aspect of the Wernicke’s or the sensorimotor cortices may be candidates for such a setup.
Ultimately, appropriate communication with the patient about every step of the operation is necessary to keep the patient relaxed and in control during these stressful moments.
Figure 5: Positioning, scalp, and bone flap design for tumors adjacent to the speech cortices. The tumor lies adjacent to the Broca’s area and extends posteriorly near the motor cortex. The bone flap is designed to encompass all the eloquent regions to be mapped. It should expose at least 2 cm of the cortex around the perimeter of the tumor and the functional cortices. Resection is guided based on mapping data and is conducted at least 1cm away from the nearby functional cortex (bottom illustration).
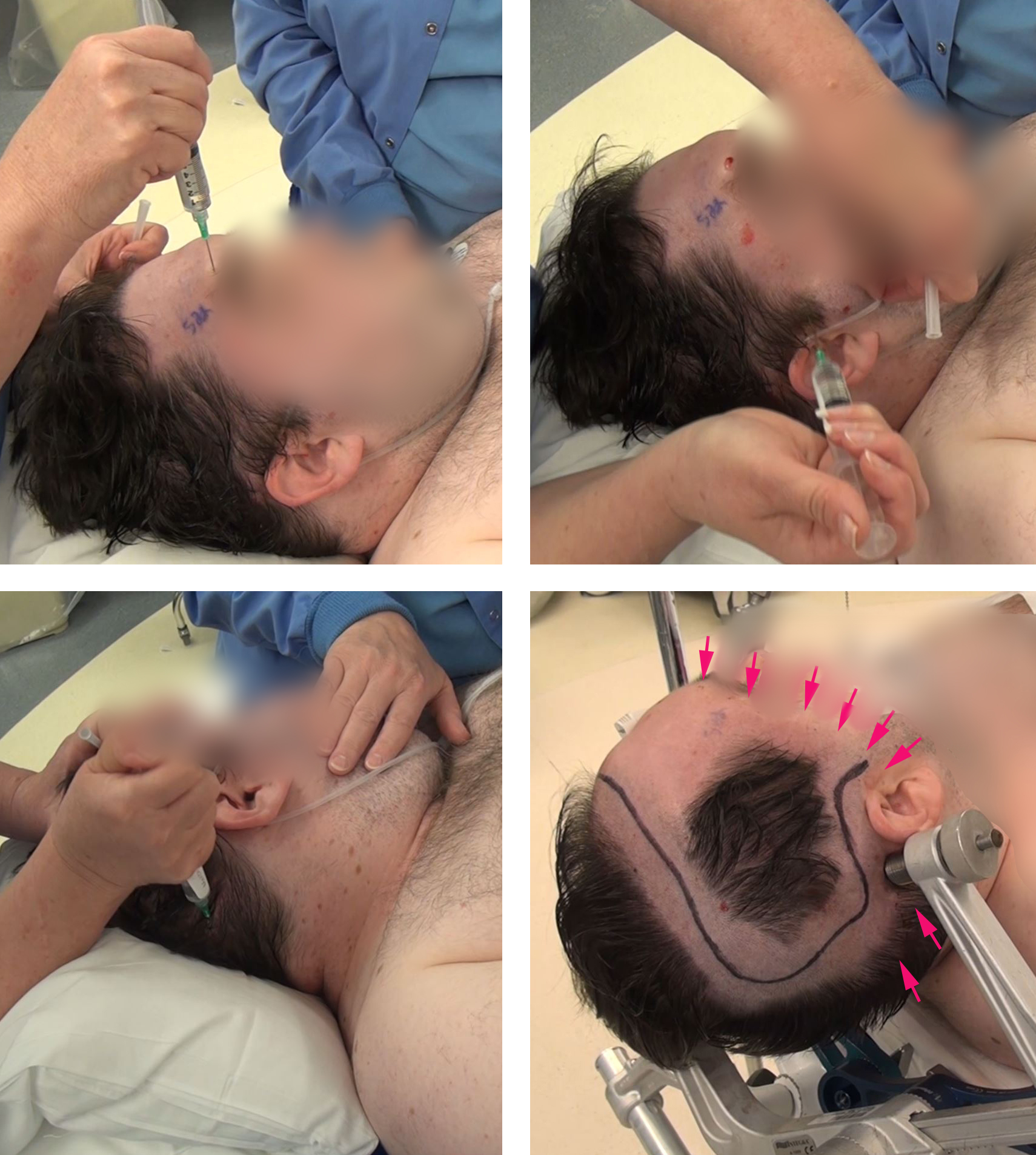
Figure 6: I use local and regional scalp anesthesia/block generously before placement of the skull clamp. The major nerve trunks supplying the ipsilateral scalp are blocked. Using generous amounts of local anesthetic (lidocaine 0.5% and Marcaine 0.25%,) I block the supratrochlear, supraorbital, auriculotemporal, and greater and lesser occipital nerves. The sites of injection for the regional scalp block are marked in red arrows (right lower image).
A copious amount of local anesthetic is also used along the planned line of incision and pin sites. The patient may also be sedated using dexmedetomidine throughout this process.
The craniotomy exposes a wide area of cortex. The temporalis muscle is repeatedly blocked for patient comfort. The surrounding brain should be exposed around the perimeter of the tumor and the adjacent cortices undergoing mapping.
Before the bone flap is elevated, local anesthetic is injected through the burr holes to irrigate the epidural space. This maneuver can potentially decrease the significant pain caused by dissecting the well-innervated and sensitive dura during the elevation of the bone flap. Once the bone flap is elevated, the dura should be blocked around the middle meningeal artery. The patient should be warned in advance of these maneuvers. A subtemporal craniectomy using bone rongeurs should be avoided because this method can be very painful.
Upon elevation of the bone flap, all sedative medications are stopped to allow the patient to awaken and be ready for cortical stimulation while the dural opening is completed. The head of the patient is raised to facilitate venous drainage and assist with cerebral relaxation.
Mapping Technique
Before any stimulation takes place, iced Ringer’s solution should be immediately available to help with a rapid cessation of seizures, if necessary. Use of ice-cold irrigation is quite effective in seizure cessation induced by cortical stimulation, obviating a need to use intravenous agents that can complicate patient’s cooperation for mapping. Prolonged generalized seizures can place the patient’s airway at risk while the head is turned and held in the skull clamp. Methohexital (Brevital) can be used if cold saline is not immediately interrupting seizure activity.
Under awake conditions, stimulation may start at 1.5 mA and increase in 1-2 mA increments (maximum of 10 mA or occasionally more) using the Ojemann stimulator. The generator should be set to deliver biphasic square wave pulses (1.25 ms per phase) in 4-second trains at 60 Hz. First, the face area is identified, and then the adjacent anterior Broca’s and posterior Wernicke’s cortices are mapped. This sequence is important to avoid confusion regarding overlapping areas containing potentially more than one function.
Stimulation is applied for 2-3 seconds at each site with tasks separated by 4–10 seconds. Each slide is presented to the patient, the results are recorded as positive or negative, and the type of language disturbance is announced to the entire team.
The bipolar electrode tips should be 1 mm in diameter and separated by 5 mm. Each cortical site is checked three times to ensure accuracy and reproducibility. While the Wernicke’s area (posterior language site-language speech) is evaluated, the slides are also presented every 4 seconds to test naming. Stimulation is applied just before the patient looks at the picture.
While the Broca’s area (anterior language site-motor speech) is being identified, the patient is asked to count from 1 to 20. Stimulation of Broca’s area will cause speech arrest without any movement in the oropharynx.
Although both negative and positive sites are marked, recent data have demonstrated the safety of limited craniotomies and cortical mapping to find “negative” locations without any function for identifying safe areas for resection (negative mapping). For negative mapping, I first determine the maximal safe stimulation parameters. To do so, I increase stimulation until afterdischarge potentials are encountered, then, the stimulation is decreased so that no such potential is again elicited. I use these stimulation settings to perform negative mapping and resect tumor in the safe areas. The results of negative mapping are based on multiple factors and anatomical considerations are also decisive.
Figure 7: Mapping technique for language is demonstrated through a left temporal craniotomy. The subdural strip performs continuous electrocorticography and monitors for afterdischarge potentials. The presence of after-discharges confirms that the stimulus/current is too high and is spreading beyond the target cortex; this finding renders the stimulation response unreliable.
Each cortical site is checked three times to ensure accuracy. The slides are presented every 4 seconds for naming. The stimulus should be delivered to the cortex just before the patient glances at the picture. Sites that induce speech arrest are tagged with labeled tickets. Although pure speech arrest and dysarthria can be distinguished by identifying mouth or oropharyngeal movements, both are important for speech function and should be preserved.
Figure 8: Tumor resection within the posteroinferior temporal lobe is illustrated. Once tumor resection begins, language exams are performed continuously because the subcortical white matter involved in language function is difficult to map/identify and a real risk of inadvertent injury during tumor resection exists. Resection borders should respect a 1cm safety margin around the functional tissue. This distance has been shown to improve preoperative language deficits, as well as the duration and permanence of postoperative language dysfunction. I have occasionally violated this rule as long as the patient’s exam is carefully and frequently monitored during this portion of the resection.
Figure 9: The dura over the skull base and falx cerebri are very sensitive and their manipulation and bipolar coagulation should be minimized to avoid significant discomfort to the patient. The innervation to these sensitive areas is marked.
Case Examples
Case 1: A young patient presented with a generalized seizure and was diagnosed with a large posterior frontal low-grade glioma.
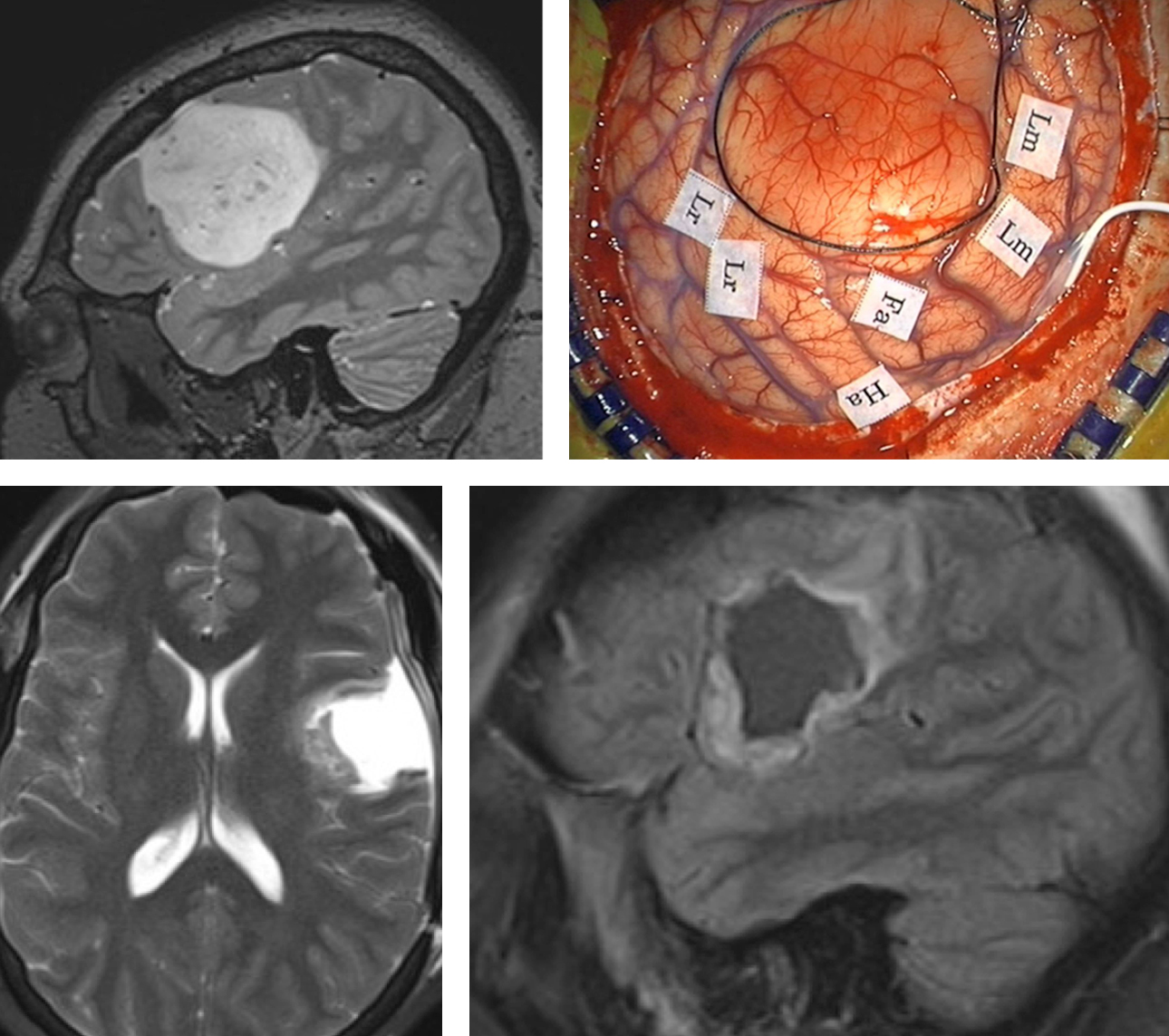
Figure 10: The preoperative MRI and intraoperative map are demonstrated (upper row). Localization of the motor speech (Lm), receptive language (Lr), Face (Fa) and hand (Ha) areas allows safe and aggressive resection of the tumor. The boundaries of the tumor are marked with a black suture. Subcortical mapping may be used at the depth of the tumor. I increase the cortical stimulation parameters by 1-2 mA to conduct subcortical mapping. During resection within close vicinity of vital cortices and tracts, frequent intraoperative exams are performed to assess the status of the patient. This immediate feedback is important to the surgeon and encourages further resection as guided by stimulation mapping data. Postoperative imaging demonstrates a reasonable resection of the mass (lower row). This patient did not suffer from the any deficit.
Case 2: Another young patient presented with a seizure and was diagnosed with a left temporal tumor.
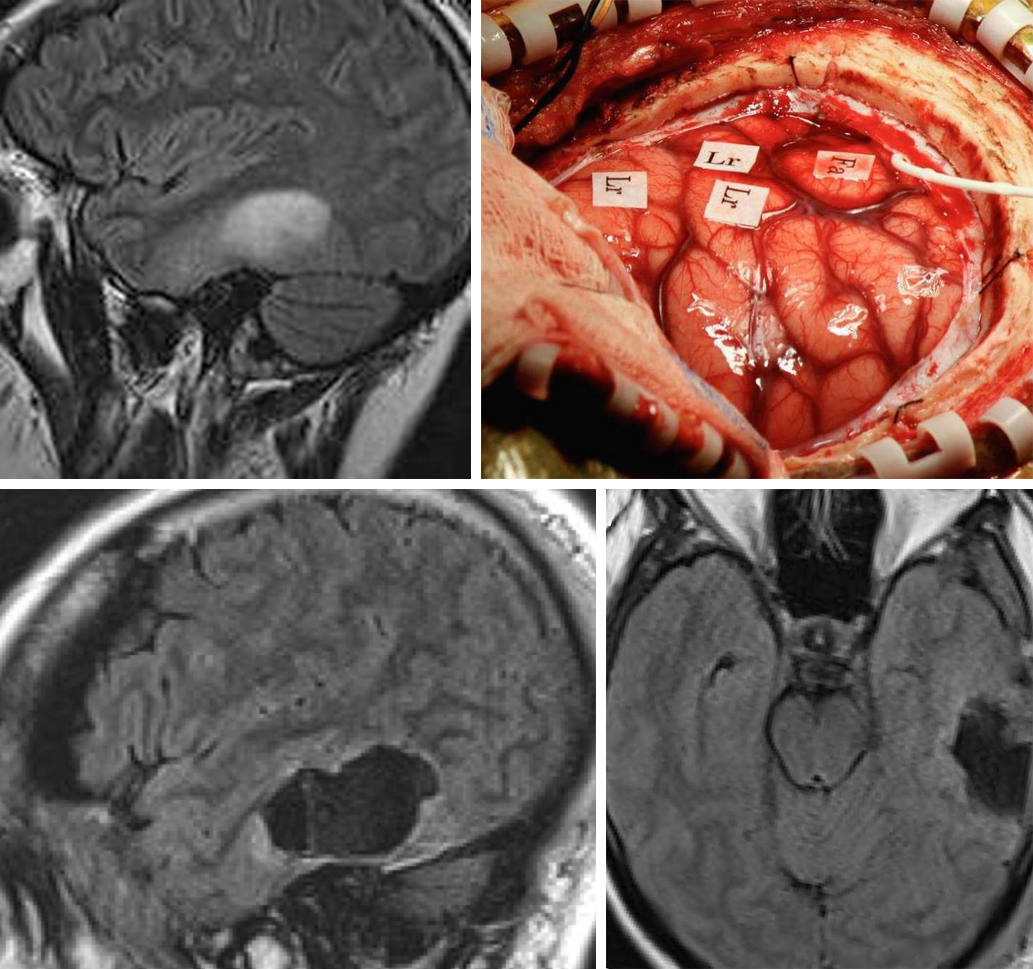
Figure 11: A left temporal low-grade glioma is located in the vicinity of the language cortices. The intraoperative map demonstrates the location of the language (Lr) and face (Fa) regions just anterior and superior to the gyri slightly expanded by the tumor (top images). Gross total resection was accomplished as guided by continuous intraoperative exams (bottom images).
Temporal Glioma: Mapping Wernicke's Area
Regardless of the function of the region that is being mapped, I use frequent intraoperative neurological exams to guide my resection strategies. Development of any slight deficit will alter my plan and/or stop further resection maneuvers.
Postoperative Considerations
Antibiotics are used for 48 hours after surgery due to the relative long duration of these mapping procedures and the limitations on sterile draping of the surgical field related to awake procedures. Anticonvulsant drugs are administered for 7 days in patients without perioperative seizures, and postoperative imaging is performed within 48 hours.
Supratherapeutic levels of anticonvulsant medications are advised due to the high risk of seizures in patients who undergo surgery of the functional cortices. Seizures delay neurologic recovery and are very disheartening to the patient’s family. The steroids are tapered slowly, especially if speech therapy is required.
The risk of temporary postoperative deficits in language function is significant. If the patient’s language is intact or mildly compromised by the end of the operation, but worsens on the first postoperative day, the opportunity for meaningful recovery is substantial. However, a significant decline at the end of the operation usually signifies an irreversible ischemic injury. The most common reason for neurologic decline on the first postoperative day is subclinical seizures.
Pearls and Pitfalls
- Patient selection is critical to the success of an awake operation. Anxious and uncooperative patients significantly increase the risks of the procedure.
- The importance of patient’s comfort and effective anesthesia cannot be overemphasized.
- The surgeon must be efficient and meticulous in mapping the eloquent cortex and expedient in performing the resection. Most patients are tolerant of awake conditions, but even the most resilient patients become inpatient and uncooperative after 4 hours of awake surgery.
- An awake procedure is truly a team effort. The surgeon must constantly communicate with the anesthesiologist, speech pathologist, and the patient.
Contributor: Richard Kim, MD
References
Berger MS, Keles GE. Tumors in eloquent areas, in Sekhar LN, Fessler RG (eds): Atlas of Neurosurgical Techniques: Brain. New York: Thieme Medical Publishers, 2011.
Keles GE, Berger MS. Functional Mapping, in Bernstein M, Berger MS (eds): Neuro-oncology: The Essentials. New York: Thieme Medical Publishers, 2011.
Naidich TP, Valavanis AG, Kubik S. Anatomic relationships along the low-middle convexity: part I – normal specimen and magnetic resonance imaging. Neurosurgery. 1995;36:517-532.
Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics. 2009;6:478-486.
Please login to post a comment.