Lateral Ventricular Tumors
Figure 1: Walter Dandy, a pioneer in intraventricular surgery, demonstrated the techniques of transcallosal and transcortical routes to the lateral ventricle (Dandy WE. The Brain. Hagerstown, MD: WF Prior 1966).
This is a preview. Check to see if you have access to the full video. Check access
Lateral Ventricular Tumor (Neurocytoma): The Interhemispheric Transcallosal Approach
Mass lesions in the lateral ventricles are rather uncommon and present a particular technical challenge because of their difficult-to-reach location and the neighboring vital diencephalic structures. These tumors are typically benign, grow at a slow rate, and are not identified until they reach a large size.
The tumor types most commonly found within the lateral ventricle are astrocytomas, ependymomas, subependymomas, meningiomas, central neurocytomas, choroid plexus papillomas, and epidermoid cysts. There are three major sites of origin for these lesions: 1) intraventricular structures, such as choroid plexus and ependyma, 2) periventricular white matter with ventricular extension, and 3) ectopic sources with ventricular metastasis.
The major vascular supply for lateral ventricular lesions includes branches of the anterior and posterior lateral choroidal arteries, which are typically reached late in resection. Venous drainage often parasitizes the ventricular wall veins. Most tumors are highly vascular and their required piecemeal resection through narrow long operative corridors involves a fair amount of bleeding; this bleeding should be minimized within the ventricular cavities as much as possible. Strategic planning to tackle the vascular pedicle early is advised.
In summary, these lesions’ characteristics of large size, deep location, and vascularity all add to the complexity of their resection complicated by a very narrow margin of technical error and an absolute need to preserve vital neurologic functions of the surrounding nonpathologic parenchyma.
Diagnosis and Evaluation
For a general discussion of diagnosis and evaluation for lateral ventricular tumors, see the Ventricular Tumors and Principles of Intraventricular Surgery chapters.
Figure 2: A large midline lateral ventricular neurocytoma (originating from the septum pellucidum) is illustrated. I approached this tumor through a left-sided transcallosal route and was unable to remove the tumor completely because of my limited operative view under the ipsilateral cingulum and the tumor’s adherence to the ventricular wall at this location. A right transcortical route through the intraparietal sulcus might have been a more appropriate alternative to reach the tumor along its long axis.
Indications for Surgery
The generally benign and slow-growing lateral ventricular lesions permit an elective approach to their surgical resection. However, patients presenting with acute obstructive hydrocephalus or intratumoral hemorrhage may require emergent surgical intervention. A complete but safe surgical resection should be planned for improvement of the patient’s symptoms and to prevent tumor progression or recurrence.
Some special tumors warrant a discussion regarding their indications for operative intervention. Management of incidentally identified lateral ventricular subependymomas is highly controversial, with an acceptable approach involving routine follow-up evaluation with surveillance imaging to assess the growth rate of the mass. If significant progression is identified or if the patient becomes symptomatic, microsurgical resection is then indicated.
Similarly, the management of incidentally identified ventricular low-grade gliomas is controversial. Routine follow-up imaging is an acceptable approach to asymptomatic lesions. As a general principle, resection of ventricular lesions demands transgression of normal cerebral tissue, and this risk must be judiciously weighed against the benefits imparted by subtotal removal of a slow growing or benign mass.
Surgical resection of an intraventricular glioblastoma is highly dependent on the patient’s neurologic status, extent of the tumor’s invasion, as well as the size and location of the lesion. Forniceal invasion associated with poor functional status is an indication for biopsy instead of aggressive surgical resection.
Preoperative Considerations
A number of factors influence the surgeon’s choice of operative corridor. Because some transection of normal brain is necessary to reach the lesion, the selection of the operative corridor plays an important role in the final outcome. The risks and benefits of each surgical route must be balanced.
I believe in the following principles for designing a safe access route to intraventricular tumors:
- minimal transgression and retraction of normal brain,
- expanded working angles along the long axis of the tumor to allow effective gross total tumor resection,
- early exposure of vital structures and access to the tumor’s blood supply, and
- the technical difficulty of the operative route.
The technical complexity of the approach is the least important consideration. Deep lesions provide a real opportunity to design innovative routes (for example, the contralateral interhemispheric transfalcine transprecuneus trajectory to reach the trigone) and minimize brain transgression. Contralateral interhemispheric approaches provide more flexible angles to access intraventricular tumors located away from the midline.
I place a lumbar drain at the beginning of the transcallosal procedures; this maneuver will markedly ease the interhemispheric dissection.
Operative Anatomy
Anatomically, the lateral ventricle may be thought of as a C-shaped capsule encompassing the thalamus and diencephalon. It is partitioned into five segmented divisions that hold important distinction during design of surgical approaches.
These divisions are the anterior (frontal) horn, body, atrium (trigone), temporal horn, and occipital horn. For a more detailed description of the relevant anatomy, please refer to the chapter on the Anatomy of the Ventricular System.
Figure 3: An interface exists between the lateral and third ventricles via the foramen of Monro. This anatomic bottleneck is bordered by the septum pellucidum, corpus callosum, caudate nucleus, thalamus, and the fornix (image courtesy of AL Rhoton, Jr).
The corpus callosum is the largest anatomic interface with the lateral ventricle. This structure is divided into four segments, from anterior to posterior: rostrum, genu, body, and splenium. Transections of the genu and splenium are avoided to prevent the risk of disconnection syndrome.
Click here to view the interactive module and related content for this image.
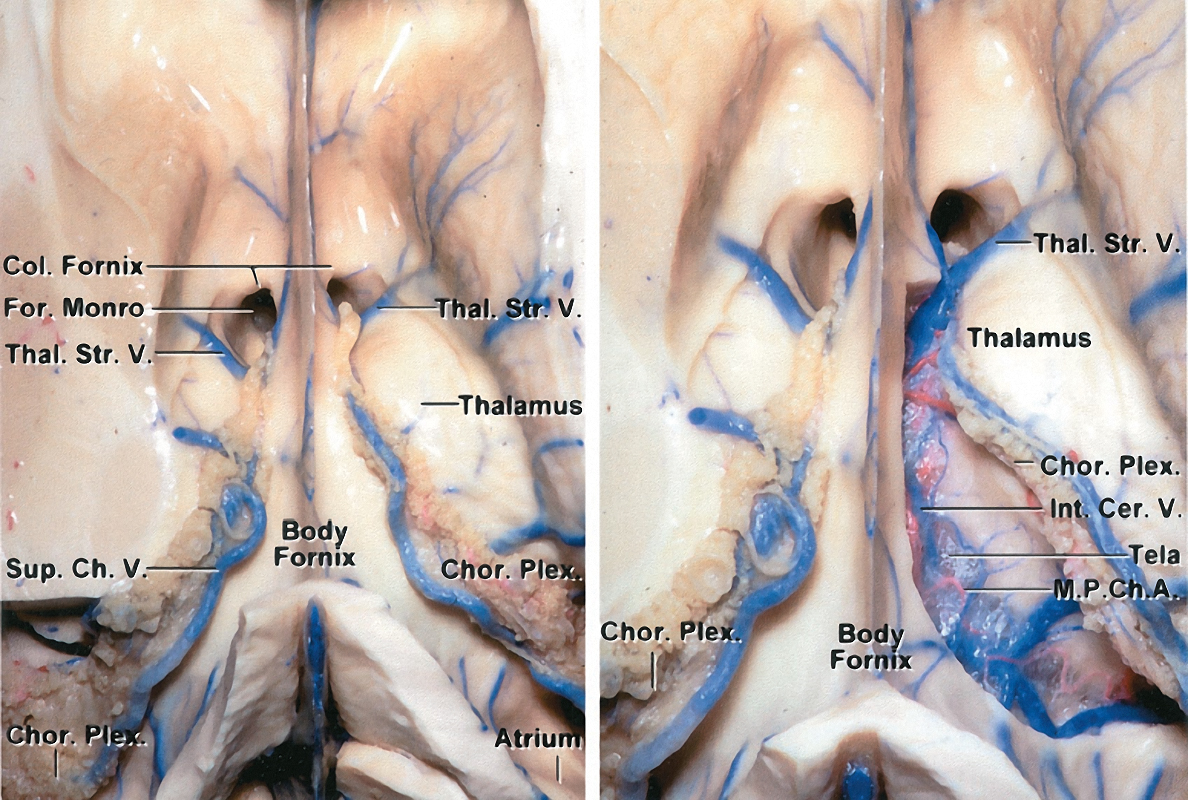
Figure 4: The fornix is one of the anatomically important structures to protect during intraventricular surgery. The fornix primarily contains projection fibers connecting the hippocampus to the hypothalamus. As bilateral fornices form a curvature along the periphery of the thalamus, both merge along its rostral surface to form the forniceal bodies. The fused fornices proceed along the periphery of the thalamus, but split adjacent to the foramen of Monro. At this level, these relatively separate structures project to the hypothalamus and mammillary bodies (left image). The right choroidal fissure has been dissected in the right image to demonstrate the structures within the roof of the third ventricle (M.P.Ch.A: Medial posterior choroidal artery)(images courtesy of AL Rhoton, Jr).
Click here to view the interactive module and related content for this image.
Figure 5: The striatum is also a key anatomic structure during ventricular surgery. The primary structure of interest is the caudate nucleus, which is anatomically segmented into three divisions: head, body, and tail. The caudate is vulnerable as it passes deep to the lateral border of the anterior horn and body of the lateral ventricle. It is also deep to the roof of the temporal horn. The genu of the internal capsule approaches the ventricular surface and directly touches the wall of the lateral ventricle immediately lateral to the foramen of Monro (left image). This occurs in the interval between the caudate nucleus and the thalamus. The disconnection of the septal vein from the thalamostriate vein will remarkably expand the transforaminal route (right image) (images courtesy of AL Rhoton, Jr).
The choroidal structures are pertinent during planning of the resection and early tumor devascularization. The choroid plexus adheres to the choroidal fissure along the medial wall of the lateral ventricle. This fissure represents the separation between the fornix and thalamus. The choroidal fissure originates adjacent to the foramen of Monro and continues along the body, atrium, and temporal horn.
Click here to view the interactive module and related content for this image.
Figure 6: The key vascular anatomy to consider during the procedure includes: anterior and posterior medial choroidal arteries (M.P.Ch.A.) (perfusing the choroid plexus,) caudate and anterior septal veins, superior choroidal vein, medial and lateral atrial veins, thalamostriate vein, inferior ventricular vein, inferior choroidal vein, and veins of the amygdala and hippocampus. The major draining veins include the internal cerebral veins and the basal veins of Rosenthal. Some of these structures are shown. The transchoroidal and interforniceal routes through the lateral ventricle are described in the top row (images courtesy of AL Rhoton, Jr).
RESECTION OF LATERAL VENTRICULAR TUMORS
The goals of the operative corridor include facilitating a least disruptive but optimal size surgical field and permitting appropriate anteroposterior and mediolateral visualization of the underlying lesion.
Different tumor types of the same size require slightly different size craniotomies and intradural trajectories because the tumors’ consistency and texture (firm versus soft) as well vascularity dictate the necessary operative angles for their gross total removal. Deep location of a tumor limits the operative space at the depth of the operative field (“cone” phenomenon). The use of dynamic retraction techniques and selection of suitable transcerebral routes (along the long axis of the tumor) promote safe and efficient handling of the underlying pathology via small and less disruptive corridors.
For more information about interhemispheric and transcortical craniotomies, please refer to the Cranial Approaches volume. Additional relevant details are briefly reviewed here.
Figure 7: Supine (left image) and lateral (right image) patient positions can provide the necessary head turn so that the sagittal suture is parallel to the floor. The axis of the patient’s cervical spine is at a 45-degree angle against the floor. The dependent hemisphere is placed “down” for exploitation of gravity retraction.
This section reiterates and expands on the introductory summary of lateral ventricular approaches in the Principles of Intraventricular Surgery chapter.
| Lesion Location | Suggested Approaches |
| Frontal horn |
|
|
Body |
|
|
Atrium or trigone |
|
|
Temporal horn |
|
|
Occipital horn |
|
The position of the lesion within the lateral ventricle determines the best direction of approach, either anterior, anterolateral, or posterior. This necessitates either a transcortical or a transcallosal pathway to reach the ventricular lumen. The choice of a particular operative pathway can be controversial and is partially accounted for by the surgeon’s preference according to his or her residency training.
The advantage of the transcortical approach is that the parasagittal veins are not a concern and the tedious interhemispheric intercingulate arachnoid dissection is not required. However, the projection fibers in the frontal lobe are disrupted during the transcortical surgery. I believe the transcallosal corridors provide more flexible operative angles, especially for larger and highly vascular lesions, and are less disruptive.
Some reports suggest that the transcortical approach increases the risk for postoperative seizures; however, this concept has not been conclusively demonstrated in subsequent studies. It is important to appropriately position and minimize the cortical or callosal incision using navigation to lessen the risk of postoperative deficits while still providing a sufficiently large passageway for exposure and resection of the lesion.
I will review the more commonly accepted operative approaches to lateral ventricular tumors based on the approaches’ ability to facilitate optimal exposure for maximal surgical resection while minimizing approach-related morbidity. Moreover, the resection maneuvers should spare the indispensable neurologic functions associated with the walls of the ventricles and deep brain nuclei.
Operative Approaches for the Anterior Horn of the Lateral Ventricle
The anterior horn is the portion of the lateral ventricle that is located anterior to the foramen of Monro. The borders of the anterior horn are the head of the caudate laterally, septum pellucidum medially, genu and rostrum of the corpus callosum rostrally and caudally. This anatomic space is optimally approached via the anterior interhemispheric transcallosal or transfrontal transcortical (via the middle frontal gyrus) approaches, detailed in the following paragraphs.
Anterior Interhemispheric Transcallosal Approach
The anterior transcallosal approach is ideal for midline lesions along the anterior two-thirds of the body of ventricle without significant lateral expansion. This approach can be attempted with the patient in the supine position and the neck flexed (“nose up”) or with the patient in the lateral position with the head rotated 90 degrees (see Figure 7).
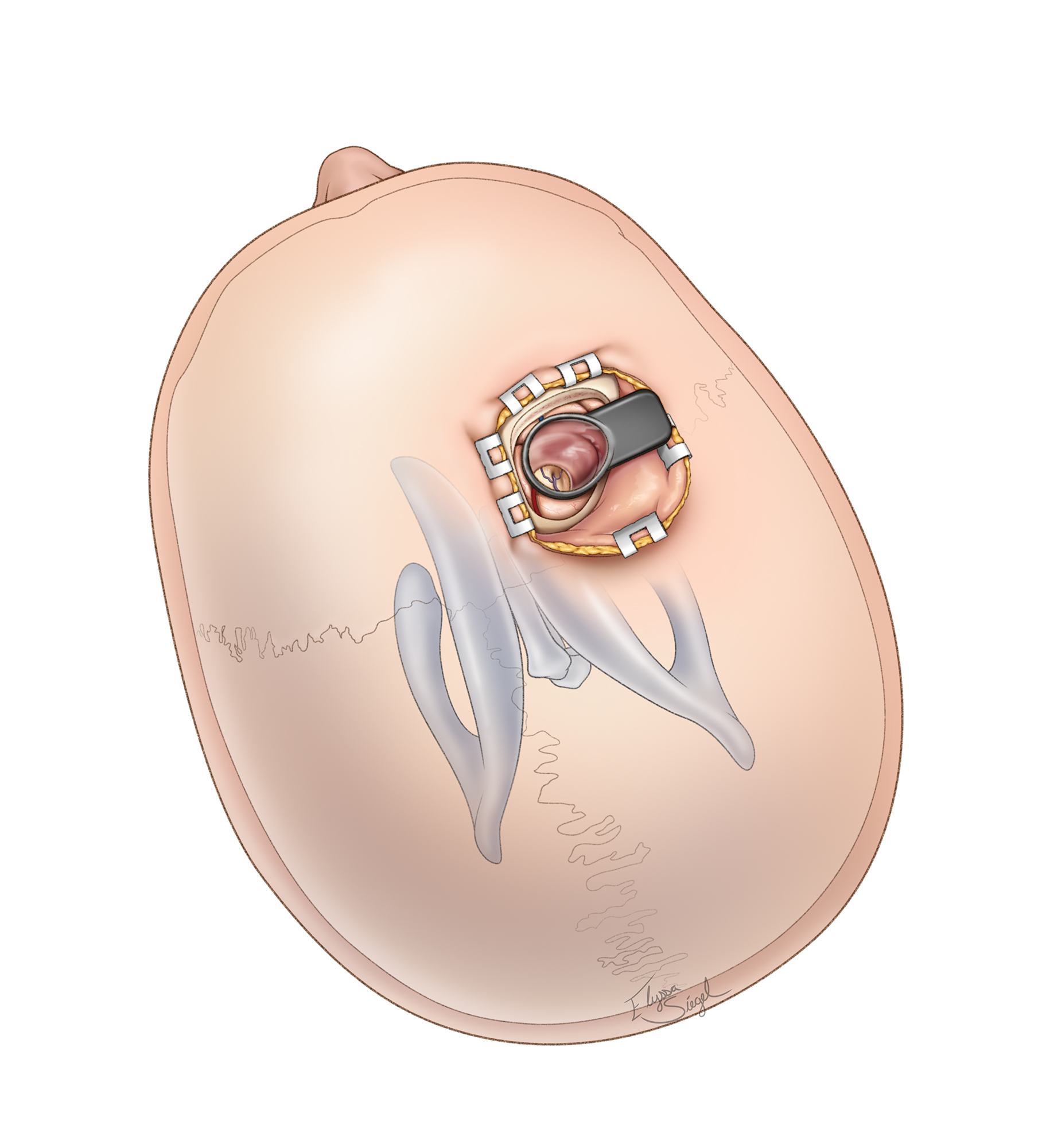
Figure 8: The craniotomy involves an abbreviated bicoronal incision posterior to the hairline, overlying or just anterior to the coronal suture. Although many surgeons advocate a horseshoe incision with the patient in the supine position and neutral head posture (left image), I use a linear incision with the patient in the supine position and the head turned 90 degrees (right image). The exact whereabouts of the incision depends on the location of the tumor; the posterior extent of the craniotomy should not reach the precentral gyrus (~3 to 4 cm behind the coronal suture). The use of cerebrospinal fluid lumbar drainage is advised so that unobstructed interhemispheric and intercingulate dissection can be facilitated.
Based on intraoperative navigation, burr holes are placed over the sagittal sinus; the craniotomy exposes the width of the sinus, but not the contralateral parasagittal dura. The dura is incised while voiding the venous sinuses and parasagittal bridging veins.
Preservation of the bridging veins is paramount and dependent on the operative trajectory. Mobilization and untethering of these veins permits medial reflection and mobilization of the dura.
Figure 9: A small right frontal craniotomy for an anterior interhemispheric approach is shown. Note the extent of exposure for the venous sinus.
Interhemispheric dissection exploits gravity retraction to mobilize the hemisphere. The cingula are commonly adherent, and gentle sharp microsurgery is necessary to avoid subpial injury. Again, navigation directs the operative trajectory at every step.
Figure 10: Two retraction sutures in the superior falx mobilize the superior sagittal sinus and expand the interhemispheric reach.
Figure 11: Patient microsurgical techniques split the intercingulate fissure widely. Pial invasion must be avoided. The cingula should not be mistaken for corpus callosum (black arrow). Both pericallosal arteries are identified (red arrows). Note the extent of intercingulate dissection to allow the hemisphere to fall away from the falx.
Figure 12: Two medium-size cotton balls (white arrows) are inserted to keep the intercingulate fissure open and obviate the need for fixed retractors.
There are often small veins on the surface of the cingula. Their gentle manipulation leads to their avulsion. The suction device should be set on low power.
Aggressive bipolar coagulation to stop bleeding should be avoided, and a small piece of cotton may be used to gently tamponade the bleeding point for a few minutes while dissection is directed elsewhere. I then return to the bleeding point and resume sharp dissection. These maneuvers avoid cortical injury that can be caused by the indiscriminate use of electrocautery. Coagulation of these small venous bleeding points often leads only to more bleeding from cortical injury. Blunt dissection should be minimized.
Wide arachnoidal dissection is ideal to mobilize the hemisphere away from the falx and obviate the need for fixed retractors. The use of CSF drainage early in surgery for brain relaxation also eliminates the need for retraction and therefore protects the parenchyma from compression and shear stress. I typically pursue the callosomarginal artery to reach the pericallosal arteries and corpus callosum. The cingula are significantly adherent and tediously difficult to microsurgically dissect. Subpial wandering will lead to cortical injury and increase the patient’s risk of postoperative seizures.
The color of the cingulum is different from the bright, glistening light yellow color of the callosum. These two structures should not be mistaken for each other. The pericallosal arteries may be displaced or adherent to each other. The sharp tip of the scissors can lead to their injury, especially if the tips of the scissors are not always in view and the artery is hidden underneath the bank of the cingulum. Mobilization of these arteries requires gentle coagulation and sharp sectioning of their small perforating arteries feeding the callosum. Forceful blunt dissection leads to perforator avulsion, placing the parent vessels at risk.
If an inadvertent perforator avulsion injury occurs and bleeding from the wall of the pericallosal artery is encountered, I place a very small piece of cotton at the bleeding point. Patience and gentle tamponade are effective and the cotton is left in place and not removed. An attempt to suture the opening in the wall of the vessel can be challenging.
A generous length of the callosum should be exposed. Navigation is used to precisely localize the most minimum length of callosotomy required to expose the middle of the lesion. The presence of hydrocephalus or tumor often leads to attenuation of the callosum. It is ideal to use the most attenuated portion for callosotomy, if possible.
I stay as lateral as possible from the pericallosal arteries in order to enter the correct ventricular chamber. An anterior callosotomy spares the genu and splenium, and is associated with a small risk of transient disconnection syndrome (mutism, apathy, akinesia, fixed gaze, disinhibition, incontinence, unilateral weakness, forced grasping, and right-left confusion). This complication is a significant risk with a splenium callosotomy.
The ventricular surface of the callosum is associated with small ependymal veins that should be coagulated before they are inadvertently avulsed. Entry into the correct ventricle is confirmed using the anatomic relationship between the thalamostriate vein and choroid plexus. Upon entering the ventricle, additional CSF drainage promotes brain relaxation. The lumbar drain remains clamped for the rest of the procedure.
If the septum pellucidum is bulging or collapsing into the exposed ventricle, it is fenestrated to decompress the contralateral ventricle. Routine fenestration in cases of bilateral ventricular obstruction is advised.
Special care must be taken to avoid injuring the genu of the internal capsule, located lateral to the foramen of Monro and thinly separated by the ventricular capsule. This is where the thalamostriate vein orients medially to anastomose with the internal cerebral vein.
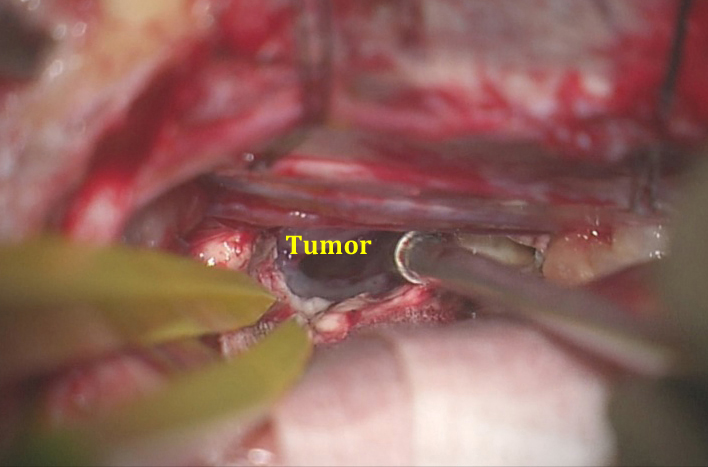
Figure 13: A small callosotomy is completed and the capsule of the tumor is exposed. I complete a restricted callosotomy over the lesion and expand the callosotomy only after the lesion is barely exposed and the tumor capsule inspected so that I can understand the exact direction of the additional callosotomy required for optimal tumor exposure. With experience, the surgeon is able to do more through less generous operative spaces using suitable working angles and dynamic retraction.
It is desirable to reach the vascular pedicle of the tumor early in surgery, although this is frequently not possible due to the presence of a large tumor is a restricted operative space. The initial devascularization is imperative for efficient tumor resection and for strict control of bleeding within the ventricles.
A small piece of cotton is used to plug the foramen of Monroe early to avoid drainage of blood into the third ventricle. Next, the tumor is debulked, dissected from the surrounding structures, and delivered using standard microsurgical techniques. The suction tip should not be placed directly on the ventricular walls. Fornices and ventricular veins are vital and should not be manipulated at the expense of gross total tumor removal.
Figure 14: After the tumor is removed, the internal cerebral veins (blue arrows) are apparent at the posterior aspect of the resection cavity where the fornices diverge.
The lateral reach of the transcallosal approach is limited, especially ipsilaterally. The surgeon’s operative blind spot is just below the ipsilateral corpus callosum. The assistance of endoscopic techniques is advised in select cases.
The line of sight of the microscope is adjusted so that the anterior and posterior poles of the tumor underneath the intact callosum are visualized.
Transfrontal Transcortical Approach (via Middle Frontal Gyrus)
The anterior transcortical approach is an acceptable technique for resection of primarily frontal horn lesions. It is especially suitable in patients with large ventricles and lesions located in the nondominant hemisphere. Unilateral tumor extensions that are not easily amenable to the midline transcallosal trajectory can be readily reached via the transcortical route.
However, this method does involve partial sacrifice of the commissural and projection fibers. Rare and mostly temporary deficits associated with this approach include speech and attention dysfunction. Patients suffering from hydrocephalus who harbor dominant hemispheric lesions may be exposed to an increased risk from this procedure.
The patient is placed supine, and the head is turned approximately 20 degrees away from the side of the lesion. Using navigation, the surgeon plans a cortical incision over the middle frontal gyrus. The corticotomy through the middle frontal gyrus, just anterior to the coronal suture, provides adequate working angles directly toward the frontal horn and the anterior body of the ventricle, as well as the foramen of Monro. The anatomic landmarks in the ventricle are the same as those for the transcallosal approach.
The anterior-posterior trajectory and contralateral reach of this route are limited. The extent of cortical injury is likely to be more extensive than the one during the transcallosal pathway. The use of a tubular retractor system is recommended to minimize the risk of this complication. Please refer to the Colloid Cyst (Transcortical Approach) chapter for the details related to the use of the transcortical transtubular route.
Figure 15: The transfrontal transcortical transtubular approach is reasonable for reaching nondominant large frontal horn lesions in the presence of hydrocephalus.
Operative Approaches for the Body of the Lateral Ventricle
The lateral ventricular body is between the foramen of Monro and the posterior margin of the septum pellucidum at the confluence of the corpus callosum and the fornix.
The optimal approaches to the lateral ventricular body include the anterior and posterior interhemispheric transcallosal, as well as the transcortical approaches. The transcallosal route provides a more posterior working space compared with its transfrontal transcortical counterpart.
The callosotomy may be completed along the body of the corpus callosum; the splenium is spared. For further details, see the Posterior Interhemispheric Transcallosal Intervenous/Paravenous Variant chapter. Although the corticotomy for the transfrontal transcortical approaches may be conducted more posteriorly, the functional cortices, including the central lobule, may be placed at risk and restrict the posterior limits of cortical incision. With more posteriorly located tumors, the transparietal transcortical route (via the superior parietal lobule) may be used.
Operative Approaches for the Atrium or Trigone of the Lateral Ventricle
The next adjacent segment within the lateral ventricle is the atrium (also referred to as the trigone), which is the site of interface between the lateral ventricular body and the occipital and temporal horns. The borders of the atrium include commissural fiber bundles from the corpus callosum (forceps major and tapetum) medially, laterally, and rostrally. The caudal border is at the collateral eminence and calcar avis.
Because of the high risk of speech and language deficits, approaching the trigone through a dominant inferior transparietal cortical incision is contraindicated. For this reason, a paramedian pathway through the superior parietal lobule is more commonly preferred. Overall, the optimal approaches to the atrium include the contralateral interhemispheric transfalcine transprecuneus, ipsilateral posterior interhemispheric transcingulate/transprecuneus, and the transparietal transsulcal (nondominant hemisphere) approaches.
I prefer the contralateral interhemispheric transfalcine transprecuneus approach to the trigone because it transgresses the least amount of normal brain. This approach is discussed in its own dedicated chapter. The risks associated with the use of this and alternative routes are discussed in that chapter.
Posterior Ipsilateral Interhemispheric Transcingulate/Transprecuneus Approach
Unlike the lateral transcortical approaches, this approach avoids contact with the posterior segment of the optic radiations along the ventricular capsule of the atrium and occipital horn. The risks of postoperative visual deficits are minimized. However, a fair amount of retraction is required to reach the lateral pole of the tumor via this approach. The bridging veins often limit the lateral scope of this operative corridor.
Transparietal Transcortical Approach (via Paramedian/Superior Parietal Lobule)
This approach optimizes access to lesions in the medial and lateral regions of the atrium. The boundaries of the superior parietal lobule include the postcentral sulcus anteriorly, as well as the intra- or intraparietal sulcus and the supramarginal gyrus inferiorly.
The cortical incision, guided by navigation, will place the pathway of dissection just lateral to the ventricle. There is some risk of acalculia, apraxia, and visual-spatial processing dysfunction from transection of the dominant hemisphere neocortex. Other risks include homonymous hemianopia and hemiparesis caused by unintended retraction injury.
Another concern is the risk of uncontrolled hemorrhage in patients with arteriovenous malformations (AVMs) and highly vascular tumors; the feeding pedicles to these lesions are not easily controlled through this approach, and they are reached during the last steps of the operation.
Contralateral Interhemispheric Transfalcine Transprecuneus Approach
Because of the above-mentioned shortcomings of other transcortical routes to the atrium, I have used the contralateral interhemispheric transfalcine transprecuneus approach, which provides an expanded interhemispheric corridor with minimal cortical transgression.
This approach exposes the tumor’s and AVMs’ vascular pedicles arising from the choroidal arteries early in surgery through its tangential operative trajectory. It may therefore minimize the amount of blood loss and the associated risks of uncontrolled bleeding into the ventricle. However, the working distance is long and the unaffected hemisphere is manipulated.
Figure 16: Contralateral interhemispheric transfalcine transprecuneus approach and its trajectory (inset image) are illustrated. The interhemispheric corridor is behind the central lobule.
Transparietal Transcortical/Transsulcal Approach (via intraparietal sulcus)
This approach is reasonable for large nondominant lesions that are not centered over the midline but fill the posterior body and the trigone of the ventricle. Compared to its transcallosal counterpart, this corridor places the operator’s working angle parallel to the long axis of the tumor, improving the opportunity for gross total resection by minimizing the operative blind spots.
Figure 17: A large meningioma within the posterior body and nondominant trigone is an excellent candidate for the intraparietal route. Note the positioning, craniotomy and typical tumor location. The slightly more anterior location of the tumor renders the contralateral interhemispheric transfalcine approach not suitable.
Operative Approaches for the Temporal Horn
The temporal horn projects anteriorly from the atrium and terminates posterior to the amygdala. The rostral borders of the temporal horn include the stria terminalis medially, and the tail of the caudate nucleus and tapetum laterally. The additional borders include the hippocampus and collateral eminence caudally and the fimbria and choroidal fissure medially.
The optimal approaches to resection of tumors within the temporal horn include the anterior temporal neocortical resection, transtemporal transcortical (via middle temporal gyrus,) transtemporal transsulcal (via occipitotemporal sulcus,) transsylvian, and transparietal transcortical (via inferior parietal lobule) approaches. The paragraphs that follow describe these surgical approaches.
Anterior Temporal Neocortical Resection
A limited anterior temporal neocortical resection can be effective for exposing masses within the anterior and mid sections of the temporal horn just at and anterior to the level of the cerebral peduncle. I favor this corridor for these regions.
Disadvantages of this approach relate to the risk of neurodeficits resulting from the sacrifice of cortical tissue. However, the proposed limited resection of the middle and inferior temporal gyri (within 3.5 cm of the temporal tip) should carry small and acceptable risks even within the dominant lobe. For more details, refer to the Anteromedial Temporal Lobectomy chapter.
Figure 18: A conservative anteromedial temporal resection provides an excellent exposure of the anterior temporal horn.
Transtemporal Transcortical Approach (via the Middle Temporal Gyrus)
A corticotomy within the dominant middle temporal gyrus has a high risk of causing speech dysfunction. The variations in the location of speech cortices in the dominant hemisphere demand cortical stimulation mapping for reliable localization of language. An approach through the nondominant hemisphere avoids this risk.
The amount of required retraction often inflicts more injury to both the middle and inferior temporal gyri than expected.
Transtemporal Transsulcal (via the Occipitotemporal Sulcus)
This transsulcus approach is useful for lesions located within the mid section of the temporal horn. The major disadvantage of this approach is the risk of postoperative visual field deficits, most commonly a superior quadrant field defect. This approach is not ideal, especially for surgeries in the dominant hemisphere. Therefore, the benefits and risks of this approach must be considered preoperatively.
I do not favor this trajectory. I prefer the use of small anterior temporal neocortical resection and a tangential operative corridor to the middle portion of the temporal horn. For more posteriorly situated lesions near the area of the calcar avis, I use the supracerebellar transtentorial transparahippocampal trajectory.
Transsylvian Approach
The transsylvian approach can also be used for accessing the anterior temporal horn. Historically, this approach was implemented for amygdalohippocampectomy; for more details, see selective amygdalohippocampectomy.
The major advantage of this approach pertains to dominant amygdala, anterior hippocampal, and parahippocampal limbic tumors that extend into the temporal horn. For these lesions, the transsylvian approach permits resection without transgression of the dominant neocortex of the superior, middle, and inferior temporal gyri.
I use this approach sparingly for small dominant amygdalar and anterior hippocampal lesions. This approach is challenging because the middle cerebral artery branches must be mobilized and manipulated within the fissure with the attended risks of vascular injury or spasm. In addition, the working corridor is limited to the anterior temporal horn and is less flexible for lesions extending to the mid section of the horn. Overall, there are limited indications for the use of this corridor.
Figure 19: The transsylvian corridor is restrictive and places the middle cerebral artery branches at risk. This sketch demonstrates resection of the right amygdala and anterior hippocampus through this route. The oculomotor nerve is apparent.
Transparietal Transcortical Approach (via Inferior Parietal Lobule)
Disadvantages of this approach are the neurodeficits that can result from dissection of the associated cortical fibers. Approaching through the dominant temporoparietal junction can result in dyslexia, agraphia, acalculia, visual field deficits, and finger agnosia due to transgression of the angular gyrus.
In the nondominant hemisphere, the same approach can potentially result in impaired memory of visual information, neglect, and visual field deficits. This approach in the nondominant hemisphere is reasonable for giant tumors filling the lateral ventricle.
Operative Approaches for the Occipital Horn
The occipital horn projects posteriorly from the atrium. Its borders include the commissural fibers of the corpus callosum laterally and rostrally, bulge of splenium and Calcar avis medially, and the collateral trigone caudally.
The optimal surgical approach for lesions within the occipital horn is debated because of the overlying highly functional medial calcarine cortex. Any transcortical approach through the occipital lobe has a high likelihood of causing a permanent homonymous visual field deficit.
Posterior Interhemispheric Transcortical Approach
The approach is favored for lesions that have already compromised vision. The anterior occipital horn is readily exposed.
Occipital Neocortical Resection
The transoccipital approach is performed using an incision at the superior occipital gyrus, and it thereby transects the optic radiations. This approach should be used only if the patient is suffering from irreversible preoperative homonymous deficit.
INTRADURAL RESECTION TECHNIQUES
Upon the surgeon’s entry into the ventricle, the topography of the tumor capsule in relation to the ventricular wall is delineated. This may be impossible in the case of a large tumor that fills most of the corresponding section of the chamber.
The operator should make every attempt to reach the vascular pedicle of the tumor early. The choroid plexus is a good landmark to find the feeding choroidal vessels to meningiomas. Gliomas and neurocytomas may not own a well-defined vascular pedicle, but typically have an identifiable point of origin (i.e., septum pellucidum) seen on preoperative imaging.
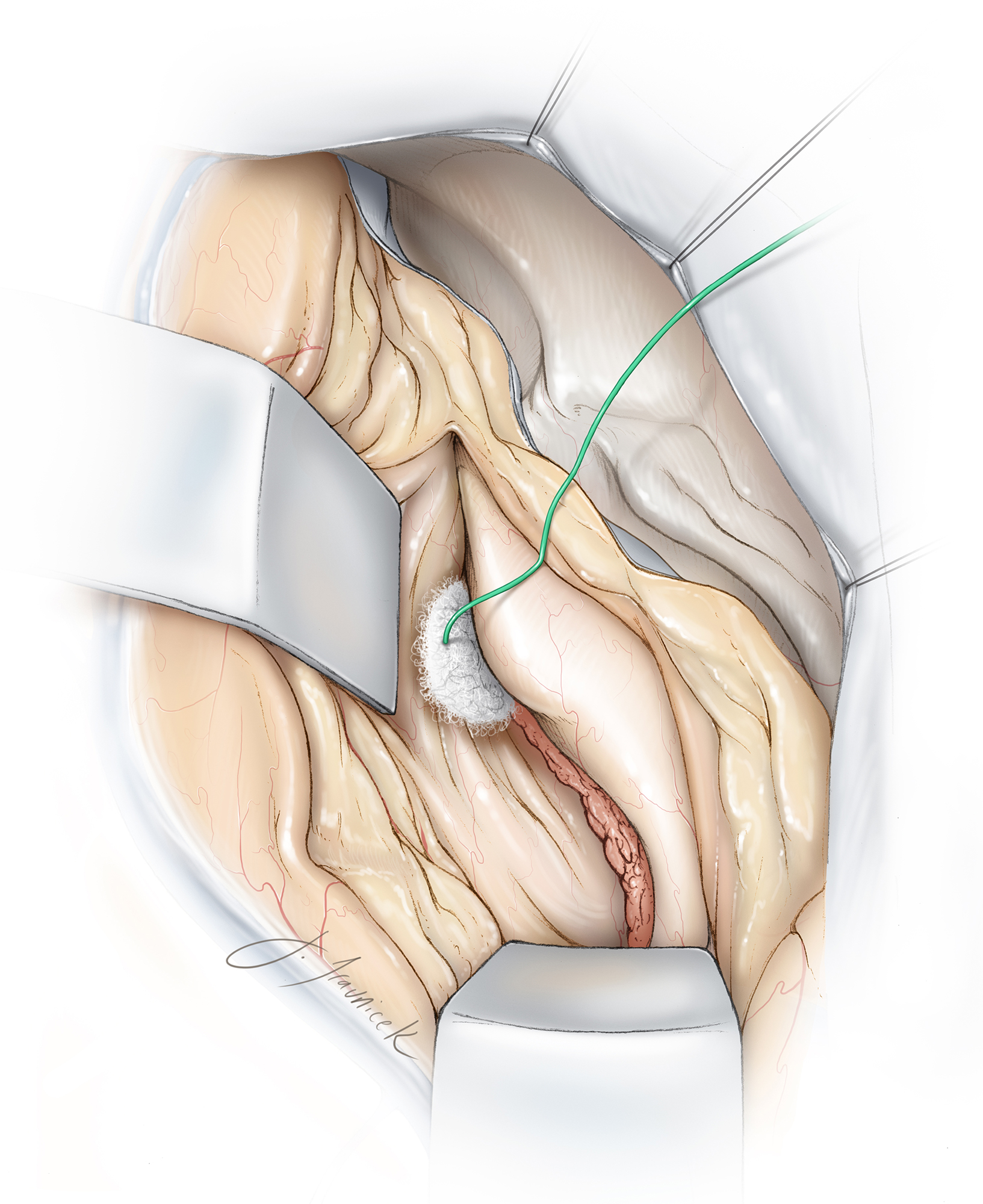
Figure 20: The transparietal transcortical/transsulcal approach (via intraparietal sulcus) is used to reach a large atrial meningioma (see Figure 17.) The tumor is gently mobilized to expose the feeding choroidal vessels along the choroid plexus (upper inset.) Coagulation of the feeding vessels (lower inset) dramatically improves operative efficiency during the later stages of the operation.
Intracapsular tumor decompression is the next key step to allow mobilization of the tumor capsule. The walls of the ventricle often do not adhere to the tumor, and mobilization of the capsule, rather than handling of the wall, is necessary to safely reveal the normal anatomy. Excessive manipulation of the ventricular wall is a significant source of postoperative morbidity in intraventricular surgery.
Figure 21: I always look for hidden vital neurovascular structure while working in deep operative corridors with multiple blind spots. In these illustrations, the tumor is carefully dissected from deep internal cerebral veins. The draining veins of the tumor are isolated and transected (inset).
Personal Reflections
While working in the lateral ventricle, I plug the foramen of Monro using a small cottonoid patty to avoid dissemination of blood and tumor into the third ventricle.
The involvement of the veins and fornices with the tumor often determines whether gross total resection is feasible. Although the septal veins are dispensable, the thalamostriate and internal cerebral veins are vital to the diencephalon. The use of an ultrasonic aspirator facilitates piecemeal tumor removal in layers. Traction on an inadequately decompressed tumor leads to unintended ventricular wall injury.
Any bleeding that ensues should be quickly controlled. However, indiscriminate coagulation should be minimized to avoid heat injury to normal structures. Ample irrigation is used to keep the cavities clean and easily identifiable while minimizing direct suctioning on the walls. I always look for the vital structures that may be in my blind spot (near or at the bottom of the tumor capsule). I would rather say “there it is” and may be wrong, but hope I never say “there it was” and be right.
The length of surgery is long, and an uncomfortable arm posture can lead to operator’s fatigue. However, the surgeon must remain composed and patient. Pulling on the tumor capsule without its complete detachment around all its corners leads to disappointing results.
Because of the limited working space at the depth of the operative field, it is not uncommon for a surgeon to unintentionally leave some tumor fragments behind. Therefore, it is best to spend a reasonable amount of time after resection to confirm the extent of removal. The use of endoscopy is encouraged.
Closure
The use of hemostatic materials in the ventricle should be minimized as they can cause acute hydrocephalus postoperatively. After resection is complete, the cavity is generously irrigated and meticulous hemostasis achieved. All cotton patties are removed and the ventricular cavities are confirmed to be communicating freely. An external ventricular drain is placed in the resection cavity and tunneled subcutaneously during closure.
I do not insist on a watertight dural closure during supratentorial surgery, even if the ventricular system is entered.
Postoperative Considerations
For a more detailed description of postoperative considerations and complications following ventricular tumor resection, see the Principles of Intraventricular Surgery chapter.
Based on the commonality of ependymomas in the lateral ventricles, the key considerations regarding follow-up for this tumor type is discussed. Although unlikely, if malignant features are found during histopathologic examination of the tumor mass and radical gross total resection was not achieved, as shown on postoperative imaging, application of radiotherapy and spinal imaging is indicated.
Pearls and Pitfalls
- Appropriate patient selection is the first important step in ventricular surgery. Design of suitable operative corridors to limit brain transgression is the next important step for maximizing outcomes.
- Manipulation of the ventricular walls is a significant cause of complications and should be minimized.
Contributor: Benjamin K. Hendricks, MD
Please login to post a comment.